
The Greenhouse Effect and Global Warming
Although seasons come and go, on average the earth’s climate is very steady. To maintain this stability, the earth must radiate thermal energy—electromagnetic waves—back into space at exactly the same average rate that it receives energy from the sun. Because the earth is much cooler than the sun, its thermal radiation is long-wavelength infrared radiation that we cannot see. A straightforward calculation using Stefan's law finds that the average temperature of the earth should be –18°C, or 0°F, for the incoming and outgoing radiation to lie in balance.
This result is clearly not correct; at this temperature, the entire earth would be covered in snow and ice. The measured global average temperature is actually a balmier 15°C, or 59°F. The straightforward calculation fails because it neglects to consider the earth’s atmosphere. At visible wavelengths, as the figure shows, the atmosphere has a wide “window” of transparency, but this is not true at the infrared wavelengths of the earth’s thermal radiation. The atmosphere lets in the visible radiation from the sun, but the outgoing thermal radiation from the earth sees a much smaller “window.” Most of this radiation is absorbed in the atmosphere.
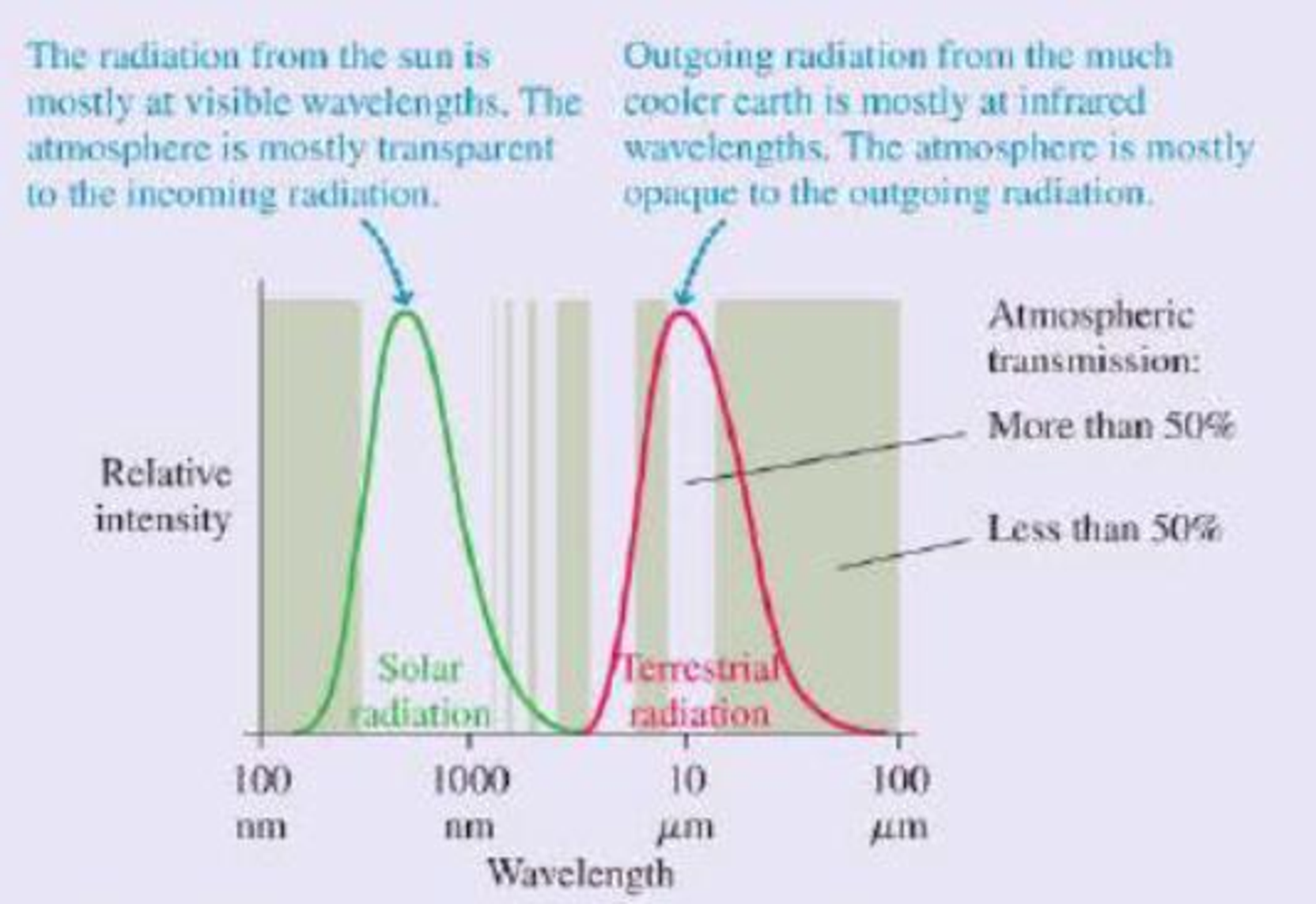
Thermal radiation curves for the sun and the earth. The shaded bands show regions for which the atmosphere is transparent (no shading) or opaque (shaded) to electromagnetic radiation.
Because it’s easier for visible radiant energy to get in than for infrared to get out, the earth is warmer than it would be without the atmosphere. The additional warming of the earth’s surface because of the atmosphere is called the greenhouse effect. The greenhouse effect is a natural part of the earth’s physics; it has nothing to do with human activities, although it’s doubtful any advanced life forms would have evolved without it.
The atmospheric gases most responsible for the greenhouse effect are carbon dioxide and water vapor, both strong absorbers of infrared radiation. These greenhouse gases are of concern today because humans, through the burning of fossil fuels (oil, coal, and natural gas), are rapidly increasing the amount of carbon dioxide in the atmosphere. Preserved air samples show that carbon dioxide made up 0.027% of the atmosphere before the industrial revolution. In the last 150 years, human activities have increased the amount of carbon dioxide by nearly 50%, to about 0.040%. By 2050, the carbon dioxide concentration will likely increase to 0.054%, double the pre-industrial value, unless the use of fossil fuels is substantially reduced.
Carbon dioxide is a powerful absorber of infrared radiation. And good absorbers are also good emitters. The carbon dioxide in the atmosphere radiates energy back to the surface of the earth, warming it. Increasing the concentration of carbon dioxide in the atmosphere means more radiation: this increases the average surface temperature of the earth. The net result is global warming.
There is strong evidence that (he earth has warmed nearly 1°C in the last 100 years because of increased greenhouse gases. What happens next? Climate scientists, using sophisticated models of the earth’s atmosphere and oceans, calculate that a doubling of the carbon dioxide concentration will likely increase the earth’s average temperature by an additional 2°C (≈ 3°F) to 6°C (≈9°F) There is some uncertainty in these calculations; the earth is a large and complex system. Perhaps the earth will get cloudier as the temperature increases, moderating the increase. Or perhaps the arctic ice cap will melt, making the earth less reflective and leading to an even more dramatic
But the basic physics that leads to the greenhouse effect, and to global warming, is quite straightforward. Carbon dioxide in the atmosphere keeps the earth warm; more carbon dioxide will make it warmer. How much warmer? That’s an important question, one that many scientists around the world are attempting to answer with ongoing research. But large or small, change is coming. Global warming is one of the most serious challenges facing scientists, engineers, and all citizens in the 21st century.
The following questions are related to the passage “The Greenhouse Effect and Global Warming” on the previous page.
The thermal radiation from the earth’s surface peaks at a wavelength of approximately 10 μm. What is the energy of a photon at this wavelength?
- A. 2.4 eV
- B. 1.2 eV
- C. 0.24 eV
- D. 0.12eV
Want to see the full answer?
Check out a sample textbook solution
Chapter P Solutions
College Physics: A Strategic Approach (3rd Edition)
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Anatomy & Physiology (2nd Edition)
Anatomy & Physiology (6th Edition)
- Hello, please help with inputing trial one into the equation, I just need a model for the first one so I can answer the rest. Also, does my data have the correct sigfig? Thanks!arrow_forwardFind the current in the R₁ resistor in the drawing (V₁=16.0V, V2=23.0 V, V₂ = 16.0V, R₁ = 2005, R₂ = and R₂ = 2.705) 2.3052 VIT A www R www R₂ R₂ Vaarrow_forwardWhich of the following laws is true regarding tensile strength? • tensile strength T ①Fbreak = Wtfest Piece thickness rate (mm) ②T = test piece width rabe (mm) Fbreak break watarrow_forward
- No chatgpt plsarrow_forwardNo chatgpt plsarrow_forwardYou hold a spherical salad bowl 85 cm in front of your face with the bottom of the bowl facing you. The salad bowl is made of polished metal with a 40 cm radius of curvature. Where is the image of your 2.0 cm tall nose located? What is image's size, orientation, and nature. I keep getting the answer -26.2, but it keeps saying it is wrong. I just want to know what i'm doing wrong.arrow_forward
- A converging lens with a focal length of 6.70 cm forms an image of a 4.60 mm tall real object that is to the left of the lens. The image is 1.50 cm tall and erect. Where are the object and image located? Is the image real or virtual? Please show all stepsarrow_forwardNo chatgpt pls will upvotearrow_forwardneed help part earrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax
AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax





