
a)
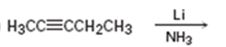
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3.
b)
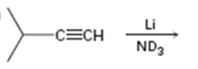
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from deuteratedammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from the solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3.
c)
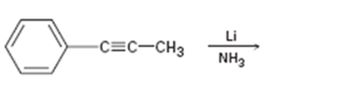
Interpretation:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from ammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from ammonia solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- 2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forward
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
- Suppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


