
a)
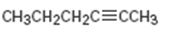
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
b)
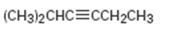
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of alkynes is an efficient method for preparing higher alkanes. Acetylene upon alkylation gives a terminal alkyne while further alkylation of a terminal alkyne leads to the formation of an internal alkyne. The actylide anion, being nucleophilic in nature, displaces the halide ion when treated with alkyl halide and gets itself attached to the alkyl group to yield a terminal alkyne. Only primary alkyl halides can be used in the reaction because when secondary and tertiary alkyl halides are used elimination of a hydrogen halide occurs instead of substitution.
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
c)
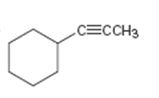
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of alkynes is an efficient method for preparing higher alkanes. Acetylene upon alkylation gives a terminal alkyne while further alkylation of a terminal alkyne leads to the formation of an internal alkyne. The actylide anion, being nucleophilic in nature, displaces the halide ion when treated with alkyl halide and gets itself attached to the alkyl group to yield a terminal alkyne. Only primary alkyl halides can be used in the reaction because when secondary and tertiary alkyl halides are used elimination of hydrogen halide occurs instead of substitution.
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule Ο C=O common name (not the IUPAC name) H ☐ H3N CH₂OH 0- C=O H NH3 CH₂SH H3N ☐ ☐ X Garrow_forward(Part A) Provide structures of the FGI products and missing reagents (dashed box) 1 eq Na* H* H -H B1 B4 R1 H2 (gas) Lindlar's catalyst A1 Br2 MeOH H2 (gas) Lindlar's catalyst MeO. OMe C6H1402 B2 B3 A1 Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forwardClassify each of the amino acids below. Note for advanced students: none of these amino acids are found in normal proteins. X CH2 H3N-CH-COOH3N-CH-COO- H3N-CH-COO CH2 CH3-C-CH3 CH2 NH3 N NH (Choose one) ▼ (Choose one) S CH2 OH (Choose one) ▼ + H3N-CH-COO¯ CH2 H3N CH COO H3N-CH-COO CH2 오오 CH CH3 CH2 + O C CH3 O= O_ (Choose one) (Choose one) ▼ (Choose one) Garrow_forward
- Another standard reference electrode is the standard calomel electrode: Hg2Cl2(s) (calomel) + 2e2 Hg() +2 Cl(aq) This electrode is usually constructed with saturated KCI to keep the Cl- concentration constant (similar to what we discussed with the Ag-AgCl electrode). Under these conditions the potential of this half-cell is 0.241 V. A measurement was taken by dipping a Cu wire and a saturated calomel electrode into a CuSO4 solution: saturated calomel electrode potentiometer copper wire CuSO4 a) Write the half reaction for the Cu electrode. b) Write the Nernst equation for the Cu electrode, which will include [Cu2+] c) If the voltage on the potentiometer reads 0.068 V, solve for [Cu²+].arrow_forward2. (Part B). Identify a sequence of FGI that prepares the Synthesis Target 2,4-dimethoxy- pentane. All carbons in the Synthesis Target must start as carbons in either ethyne, propyne or methanol. Hint: use your analysis of Product carbons' origins (Part A) to identify possible structure(s) of a precursor that can be converted to the Synthesis Target using one FGI. All carbons in the Synthesis Target must start as carbons in one of the three compounds below. H = -H H = -Me ethyne propyne Synthesis Target 2,4-dimethoxypentane MeOH methanol OMe OMe MeO. OMe C₂H₁₂O₂ Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forwardDraw the skeletal ("line") structure of the smallest organic molecule that produces potassium 3-hydroxypropanoate when reacted with KOH. Click and drag to start drawing a structure. Sarrow_forward
- draw skeletal structures for the minor products of the reaction.arrow_forward1. Provide missing starting materials, reagents, products. If a product cannot be made, write NP (not possible) in the starting material box. C7H12O Ph HO H 1) 03-78 C 2) Me₂S + Ph .H OH + 2nd stereoisomer OH Ph D + enantiomer cat OsO 4 NMO H2O acetonearrow_forwardPlease note that it is correct and explains it rightly:Indicate the correct option. The proportion of O, C and H in the graphite oxide is:a) Constant, for the quantities of functional groups of acids, phenols, epoxy, etc. its constants.b) Depending on the preparation method, as much oxidant as the graphite is destroyed and it has less oxygen.c) Depends on the structure of the graphic being processed, whether it can be more tridimensional or with larger crystals, or with smaller crystals and with more edges.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


