
a)
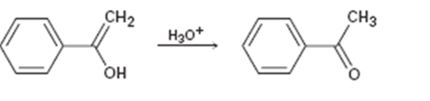
Interpretation:
Given that the final step in the hydration of an
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation ion then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
b)
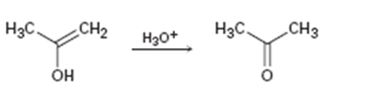
Interpretation:
Given that the final step in the hydration of an alkyne under acidic conditions is the tautomerization of the enol intermediate to give the corresponding ketone. The mechanism involves protonation followed by deprotonation. Based on this information a mechanism for the tautomerization reaction shown is to be drawn.
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
c)
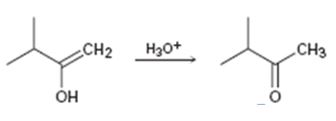
Interpretation:
Given that the final step in the hydration of an alkyne under acidic conditions is the tautomerization of the enol intermediate to give the corresponding ketone. The mechanism involves protonation followed by deprotonation. Based on this information a mechanism for the tautomerization reaction shown is to be drawn.
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- Y= - 0.039 (14.01) + 0.7949arrow_forwardSuppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forward
- Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forwardChoose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

