
Concept explainers
Draw structures corresponding to the following names:
(a) 3, 3-Dimethyl-4-octyne
(b) 3-Ethyl-5-methyl-1, 6, 8-decatriyne
(c) 2, 2, 5, 5-Tetramethyl-3-hexyne
(d) 3, 4-Dimethylcyclodecyne
(e) 3, 5-Heptadien-1-yne
(f) 3-Chloro-4, 4-dimethyl-1-nonen-6-yne
(g) 3-sec-Butyl-1-heptyne
(h) 5-tert-Buty1-2-methyl-3-octyne
a) 3,3-Dimethyl-4-octyne.
Interpretation:
The structure of 3,3-Dimethyl-4-octyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne is
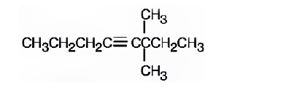
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with a triple bond between C4 & C5 and two methyl groups should be present on C3.
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne is

b) 3-Ethyl-5-methyl-1,6,8-decatriyne.
Interpretation:
The structure of 3-ethyl-5-methyl-1,6,8-decatriyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne is
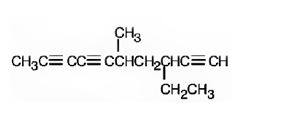
Explanation of Solution
The name shows that the longest carbon chain should contain ten carbons with three triple bonds, one between C1 & C2, another between C6 & C7 a third one between C8 & C9, an ethyl group on C3 and a methyl group on C5.
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne is

c) 2,2,5,5- tetramethyl-3-hexyne.
Interpretation:
The structure of 2,2,5,5- tetramethyl-3-hexyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne is
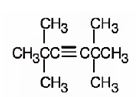
Explanation of Solution
The name shows that the longest carbon chain should contain six carbons with a triple bond between C3 & C4 and four methyl groups, two on C2 and another two on C5.
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne is

d) 3,4-Dimethylcyclodeceyne.
Interpretation:
The structure of 3,4-Dimethylcyclodeceyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne is
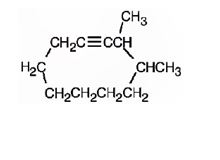
Explanation of Solution
The name shows that the compound contains a ring made up of ten carbon atoms with a triple bond attached to two methyl groups on C3 and C4.
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne is

e) 3,5-Heptadiene-1-yne.
Interpretation:
The structure of 3,5-heptadiene-1-yne is to be drawn.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To draw:
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne is
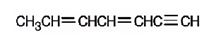
Explanation of Solution
The name shows that the longest carbon chain should contain seven carbons with a triple bond between C1 & C2 and two double bonds, one between C3 & C4 and another between C5 & C6.
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne is
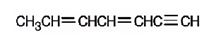
f) 3-Chloro-4,4-dimethyl-1-nonene-6-yne.
Interpretation:
The structure of 3-chloro-4,4-dimethyl-1-nonene-6-yne is to be drawn.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To draw:
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne is
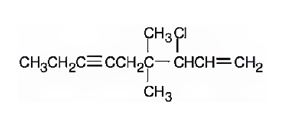
Explanation of Solution
The name shows that the longest carbon chain should contain nine carbons with a triple bond between C6 & C7, one double bond between C1 & C2, a chlorine atom on C3 and two methyl groups on C4.
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne is

g) 3-sec-Butyl-1-heptyne.
Interpretation:
The structure of 3-sec-Butyl-1-heptyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne is
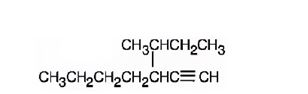
Explanation of Solution
The name shows that the longest carbon chain should contain seven carbons with a triple bond between C1 & C2 and a sec-butyl group on C3.
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne is

h) 5-tert-Butyl-2-methyl-3-octyne.
Interpretation:The structure of 5-tert-Butyl-2-methyl-3-octyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne is
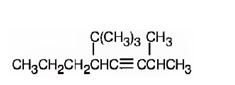
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with a triple bond between C3 & C4, a tert-butyl group on C5 and a methyl group on C2.
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne is

Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- K Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forward
- Please draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
- Name the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





