
a)
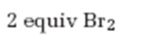
Interpretation:
The product formed when 2-hexyne reacts with 2 equivalents of bromine.
Concept introduction:
In addition reactions,
To give:
The product formed when 2-hexyne reacts with two molar equivalents of bromine.
b)
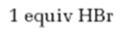
Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with two molar equivalents of a reagent yield the derivatives of the corresponding alkane as the product. With one molar equivalent of a reagent they yield a derivative of the corresponding alkene as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with 1 equivalent of HBr.
c)

Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with excess amount of a reagent yield the derivatives of the corresponding alkane as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with excess of HBr.
d)
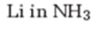
Interpretation:
The product formed when 2-hexyne reacts with Li in ammonia.
Concept introduction:
Alkynes can be reduced by treatment with hydrogen in the presence of a catalyst. The hydrogenation occurs with anti stereochemistry to give a trans- alkene as product if Li in ammonia is used.
To predict:
The product formed when 2-hexyne reacts with Li in ammonia.
e)
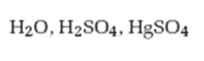
Interpretation:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4 is to be given.
Concept introduction:
When treated with H2O, H2SO4 in the presence of HgSO4 alkynes get hydrated to produce enols. The addition of water to unsymmetrical alkynes follows Markonikov regiochemistry while it cannot be applied in the case of symmetrical alkynes. The enols produced then undergo tautomerization to give a
To predict:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- Name the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forward
- Predict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forward
- For the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forwardUsing arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forward
- draw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forwardManoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

