
Concept explainers
a)
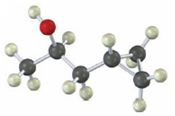
Interpretation:
Starting from any compound having four or less number of carbon atoms how the compound shown can be prepared is to be stated.
Concept introduction:
A cyclopropane ring is formed in Simmons-Smith reaction when an alkene is treated with CH2Cl2/Zn-Cu couple. Higher
To state:
Starting from any compound having four or less number of carbon atoms how the compound shown can be prepared.
b)
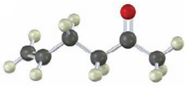
Interpretation:
Starting from any compound having four or less number of carbon atoms how the compound shown can be prepared is to be stated.
Concept introduction:
The compound shown has a keto group at C2 and a double bond between C5 & C6. The keto group can be introduced by hydration of an alkyne with aqueous H2SO4 in the presence of HgSO4. An enol obtained during hydration upon tautomerization yields the
To state:
Starting from any compound having four or less number of carbon atoms how the compound shown can be prepared.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
- Convert the following structures into a chair representation. Then conduct a chair flip. Cl a. b. C\.... оarrow_forwardAktiv Learning App Cengage Digital Learning Part of Speech Table for Assign x o Mail-Karen Ento-Outlook * + app.aktiv.com Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 17 of 30 Drawing Arrows heat 4 O M B D 5x H H Und Settings H Done :0: H Jararrow_forwardConvert the following chairs into ring representations: a. Brz b.arrow_forward
- Drawing Arrows 1 I I 1 heat 1 51 MO + Drag To Und Settings Done 0 0 Jan 31 3:5arrow_forwardDon't used hand raitingarrow_forwardGramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forward
- Don't used hand raitingarrow_forwardCHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forwardCHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




