
a)
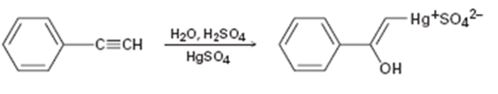
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of phenylacetylene is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of ethynylbenzene.
b)
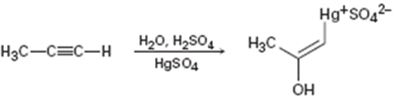
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of propyne is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of propyne.
c)
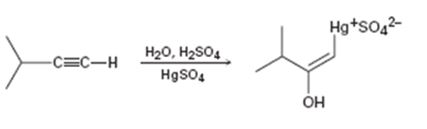
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of 3-methyl-1-butyne is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of 3-methyl-1-butyne.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- What is the preparation of 1 Liter of 0.1M NH4Cl buffer at pH 9.0 with solid NH4Cl and 0.1M NaOH. How would I calculate the math to describe this preparation? How would I use Henderson-Hasselbach equation?arrow_forwardC Predict the major products of this organic reaction. Be sure you use wedge and dash bonds when necessary, for example to distinguish between major products with different stereochemistry. : ☐ + x G C RCO₂H Click and drag to start drawing a structure.arrow_forwardFill in the blanks by selecting the appropriate term from below: For a process that is non-spontaneous and that favors products at equilibrium, we know that a) ΔrG∘ΔrG∘ _________, b) ΔunivSΔunivS _________, c) ΔsysSΔsysS _________, and d) ΔrH∘ΔrH∘ _________.arrow_forward
- Highest occupied molecular orbital Lowest unoccupied molecular orbital Label all nodes and regions of highest and lowest electron density for both orbitals.arrow_forwardRelative Intensity Part VI. consider the multi-step reaction below for compounds A, B, and C. These compounds were subjected to mass spectrometric analysis and the following spectra for A, B, and C was obtained. Draw the structure of B and C and match all three compounds to the correct spectra. Relative Intensity Relative Intensity 20 NaоH 0103 Br (B) H2504 → (c) (A) 100- MS-NU-0547 80 40 20 31 10 20 100- MS2016-05353CM 80 60 100 MS-NJ-09-3 80 60 40 20 45 J.L 80 S1 84 M+ absent राग 135 137 S2 62 164 166 11 S3 25 50 75 100 125 150 175 m/zarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raitingarrow_forwardDon't used hand raitingarrow_forwardA composite material reinforced with aligned fibers, consisting of 20% by volume of silicon carbide (SiC) fibers and 80% by volume of polycarbonate (PC) matrix. The mechanical characteristics of the 2 materials are in the table. The stress of the matrix when the fiber breaks is 45 MPa. Calculate the longitudinal strength? SiC PC Elastic modulus (GPa) Tensile strength (GPa) 400 2,4 3,9 0,065arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


