
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 56P
Propose a mechanism for the following reaction:
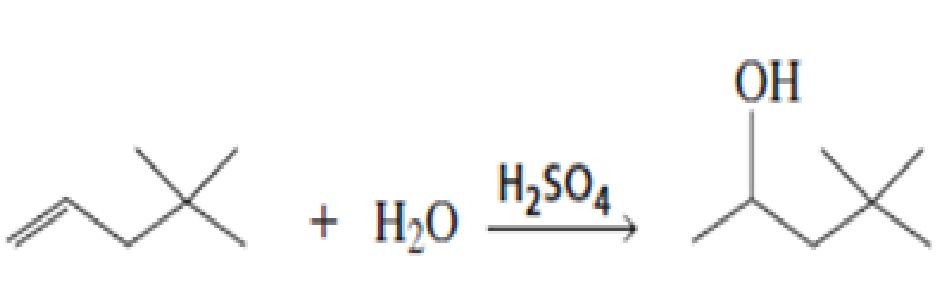
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting don't used Ai solution
Homework: Atomic Structure
This homework is due at the beginning of class next lecture period and is worth
6 points. Please place the number of protons and neutrons in the nucleus and
then put the number of electrons in the correct shell. Also give the correct
atomic mass. Also, state if the atom is an ion (cation or anion).
H*
1.
Number of protons
Number of electrons
Number of neutrons
Atomic mass
2.
26
13AI
+++
Number of protons
Number of neutrons
Number of electrons
Atomic mass
Don't used hand raiting
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help working this problem out step by step, I was trying to use my example from the txt book but all I know how to do is set it up. I need to be shown step by step as I am a visual learner. Please help me.arrow_forwardDon't used hand raitingarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
- & Calculate the molar enthalpy of combustion (A combH) of 1.80 g of pyruvic acid (CH3COCOOH; 88.1 g mol-1) at 37 °C when they are combusted in a calorimeter at constant volume with a calorimeter constant = 1.62 kJ °C-1 and the temperature rose by 1.55 °C. Given: R = 8.314 J mol −1 °C-1 and the combustion reaction: AN C3H4O3 + 2.502(g) → 3CO2(g) + 2H2O(l)arrow_forwardAn unknown salt, AB, has the following precipitation reaction:A+(aq) + B-(aq) ⇌ AB(s) the K value for this reaction is 4.50 x10-6. Draw a model that represents what will happen when 1.00 L each of 1.00 M solution of A+(aq) and 1.00M solution of B-(aq) are combined.arrow_forward5. a) Use the rules in Example 4.4 (p. 99) and calculate sizes of octahedral and tetrahedral cavities in titanium and in zirconium. Use values for atomic radii given in Fig. 9.1 (p.291). (3 points) b) Consider the formation of carbides (MC) of these metals. Which metal is able to accommodate carbon atoms better, and which cavities (octahedral or tetrahedral) would be better suited to accommodate C atoms into metal's lattice? (4 points)arrow_forward
- 2. Read paragraph 3.4 in your textbook ("Chiral Molecules"), and explain if Cobalt(ethylenediamine) 33+ shown in previous problem is a chiral species. If yes, draw projections of both enantiomers as mirror images, analogous to mirror projections of hands (below). Mirror (4 points)arrow_forward3. Borane (BH3) belongs to D3h point group. Consider the vibrational (stretching) modes possible for B-H bonds under D3h symmetry. Using the methods we used in class, construct the reducible representation I, and break it down into irreducible representations using the character table provided. Sketch those modes, indicate whether they are IR-active. (6 points) D3h E 2C3 3C2 σh 283 30% A₁' 1 1 1 1 1 1 x² + y², z² 1 -1 1 1 -1 R₂ E' 2 0 2 0 (x, y) (x² - y², xy) " A₁" 1 1 -1 A2" 1 -1 -1 1 Z E" 2 -1 0 -2 1 0 (Ry, Ry) (xz, yz)arrow_forward1. List all the symmetry elements, and assign the compounds to proper point groups: a) HCIBrC-BrCIH Cl Br H (2 points) H Br b) Pentacarbonylmanganese(I)bromide Br OEC-Mn-CEO 00- c) Phenazine (aromatic molecule, with delocalized bonding) 1 d) Cobalt(ethylenediamine)33+ (just the cation) 3+ H₂N H₂ .NH2 (CI)3 NH2 H2 H₂N. (2 points) (2 points) (2 points)arrow_forward
- Hello, I desperately need help figuring out 8-14; I also wanted to see if you would mind letting me know if I picked the right degree as my melting points on the two graphs. Please and thank you in advance! All the information is provided.arrow_forwardThe reaction: A + B ⇌ 2 C, can be represented by the equilibrium expression, KC =[C]2[A][B]=258 at 520K.When 1.00 M of C was allowed to reach equilibrium and 0.055 M of A was formed. If this reaction wasperformed at the same temperature using 0.500 M C, what would the equilibrium concentration of Abe?arrow_forward1. What is the functional group of an alcohol and a phenol? 2. Why are some alcohols soluble in water? 3. Classify each of the following alcohols as primary, secondary or tertiary. a. 3-pentanol b. 2-methyl-2-butanol c. 1-propanolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License