
(a)
Interpretation:
The
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
(b)
Interpretation:
The alkene used to prepare the given compound has to be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
(c)
Interpretation:
The alkene used to prepare the given compound has to be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Halogen addition to an alkene:
The addition reaction of halides to alkenes leads to the formation of vicinal dihalide as the product. The two halogens will be on the adjacent carbons.
Mainly
In general, the reaction can be shown as below.
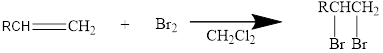
When the bromine approaches the alkene, one bromine accepts the electrons from alkene and give them to the other bromine and a cyclic bromonium ion intermediate is formed.
The formed unstable intermediate reacts with
(d)
Interpretation:
The alkene used to prepare the given compound has to be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




