
Concept explainers
(a)
Interpretation:
The product obtained from reaction
Concept Introduction:
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition of hydrogen halides to
Electrophilic addition of hydrogen halide to alkyne occurs according to the following general mechanism.
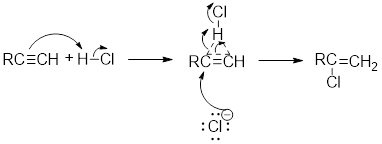
First a
(b)
Interpretation:
The product obtained from reaction
Concept Introduction:
Addition of hydrogen halides to alkynes:
Electrophilic addition of hydrogen halide to alkyne occurs according to the general mechanism.
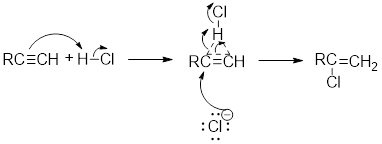
First a
(c)
Interpretation:
The product obtained from reaction
Concept Introduction:
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a
Conversion of terminal alkynes into enol: If we want to convert terminal alkyne into an enol, the presence of mercuric ion as a catalyst should be needed and the catalyst will increase the
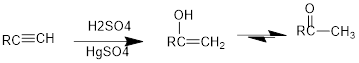
(d)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagents is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans
(e)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagent is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
(f)
Interpretation:
The product obtained from reaction
Concept Introduction:
Deprotonation: The reaction in which proton is removed from the compound using reagents is known as deprotonation.
Different reagents are used for the deprotonation and one of the common reagents is sodium amide.
Lindlar catalyst: The catalyst is used for the hydrogenation of alkynes in a syn manner. This means both hydrogen are added on the same side across the triple bond and the product obtained will be a cis product.
Sodium in liquid ammonia: The catalyst is used for the formation of trans alkenes from alkynes. Because of its more reactivity towards triple bonds, the reaction will stop at the formation of alkenes.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry, Global Edition
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





