
Concept explainers
Draw a Lewis structure for each of the following species:
- a. H2CO3
- b. CO32−
- c. CH2O
- d. CO2
(a)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
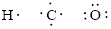
Hydrogen can form only one bond, carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
The Lewis structure can be,
 or
or 
Each atom has to achieve an octet and the existing electron has to be shared.
(b)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
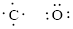
Carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
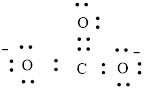 or
or 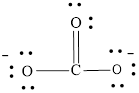
Each atom has to achieve an octet and the existing electron has to be shared.
(c)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,
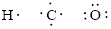
Hydrogen can form only one bond, carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
The Lewis structure can be,
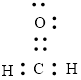 or
or 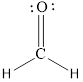
Each atom has to achieve an octet and the existing electron has to be shared.
(d)
Interpretation:
Lewis structure of
Concept Introduction:
A Lewis dot structure for an atom consists of a symbol for the element and one dot for each valence electron.
Steps in drawing a Lewis dot structure,
- Total number of valence electron has to be known.
- Atoms has to be distributed by the knowing the valency of each atom.
- Form bonds and fill the octet with lone pair of electrons.
- Formal charge if present has to be assigned.
Explanation of Solution
Given compound is
Number of valence electron of each atoms is identified and drawn as below,

Carbon can form four bonds and oxygen has six valence electrons.
Atoms have to be distributed by putting hydrogen on outside the molecule.
Here, there are two oxygen atoms, so avoiding the oxygen-oxygen single bonds.
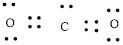 or
or 
Each atom has to achieve an octet and the existing electron has to be shared.
Want to see more full solutions like this?
Chapter 1 Solutions
Essential Organic Chemistry, Global Edition
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Biology: Life on Earth (11th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Organic Chemistry
- If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.arrow_forwardIf the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardIf the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forward
- When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forwardDoes Avogadro's number have units?arrow_forward
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
