
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 52P
- a. Which of the indicated bonds in each compound is shorter?
- b. Indicate the hybridization of the C, O, N, and halogen atoms in each of the compounds.
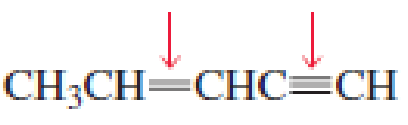
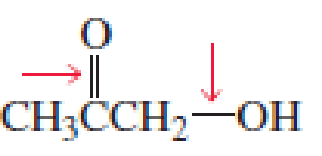
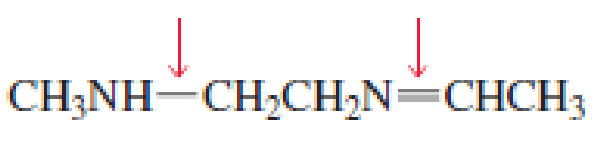
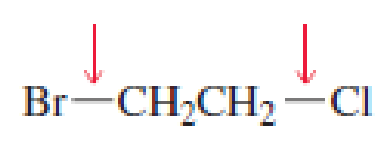
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.
a) Draw the dominant Lewis structure for the allene molecule (1,2-propyl diene, CH2CCH2 ) and use VSEPR theory to determine the molecule's geometry. In specifying the geometry give all bond angles and specify which nuclei lie in the same plane.
b) Propose a hybridization and bonding scheme for the atoms in allene. That is, specify how each of the atoms is hybridized, which atomic orbitals overlap to form bonding molecular orbitals, and the nature (i.e., sigma", pi, etc.) of these molecular orbitals.
c) Draw a plausible valence molecular orbital level diagram for allene (include only bonding MOs) based on the results from (b).
d) Based on the work from (c) deduce the valence electronic configuration of the ground state of allene.
e) Is the molecule planar or nonplanar? Explain your answer
Answer subparts c, d, and e
1.
a) Draw the dominant Lewis structure for the allene molecule (1,2-propyl diene, CH2CCH2 ) and use VSEPR theory to determine the molecule's geometry. In specifying the geometry give all bond angles and specify which nuclei lie in the same plane.
b) Propose a hybridization and bonding scheme for the atoms in allene. That is, specify how each of the atoms is hybridized, which atomic orbitals overlap to form bonding molecular orbitals, and the nature (i.e., 0", 7f, etc.) of these molecular orbitals.
c) Draw a plausible valence molecular orbital level diagram for allene (include only bonding MOs) based on the results from (b).
d) Based on the work from (c) deduce the valence electronic configuration of the ground state of allene.
e) Is the molecule planar or nonplanar? Explain your answer
A.) What is the electron geometry of IF5?
B.) What is the molecular geometry of IF5?
C.) Ignoring lone-pair effects, what is the smallest bond angle in IF5?
Chapter 1 Solutions
Essential Organic Chemistry, Global Edition
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.2 - Prob. 2PCh. 1.2 - How many valence electrons do chlorine, bromine,...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar?Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...
Ch. 1.4 - Prob. 12PCh. 1.4 - a. Draw two Lewis structures for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 16PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 18PCh. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.9 - Put a number in each of the blanks: a. ___ s...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 25PCh. 1.13 - a. Predict the relative lengths and strengths of...Ch. 1.13 - Prob. 28PCh. 1.14 - Which of the bonds in a carbonoxygen double bond...Ch. 1.14 - Caffeine is a natural insecticide, found in the...Ch. 1.14 - a. What is the hybridization of each of the carbon...Ch. 1.14 - Prob. 33PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Account for the difference in the shape and color...Ch. 1.15 - Which of the following molecules would you expect...Ch. 1 - Draw a Lewis structure for each of the following...Ch. 1 - Prob. 38PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Prob. 40PCh. 1 - Draw the condensed structure of a compound that...Ch. 1 - Prob. 42PCh. 1 - Prob. 43PCh. 1 - Draw a Lewis structure for each of the following...Ch. 1 - Prob. 45PCh. 1 - List the bonds in order from most polar to least...Ch. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Write the Kekul structure for each of the...Ch. 1 - Assign the missing formal charges.Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 51PCh. 1 - a. Which of the indicated bonds in each compound...Ch. 1 - In which orbitals are the lone pairs in nicotine?...Ch. 1 - Draw the missing lone-pair electrons and assign...Ch. 1 - Rank the following compounds from highest dipole...Ch. 1 - Prob. 56PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 58PCh. 1 - Sodium methoxide (CH3ONa) has both ionic and...Ch. 1 - a. Why is a H 8 H bond (0.74 ) shorter than a C 8...Ch. 1 - Which compound has a larger dipole moment, CHCl3...Ch. 1 - Which compound has a longer C 8 Cl bond?Ch. 1 - Prob. 63PCh. 1 - The following compound has two isomers. One isomer...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Strike-anywhere matches contain a layer of KClO3 and a layer of P4S3. The heat produced by the friction of striking the match causes these two compounds to react vigorously, which sets fire to the wooden stem of the match. KCIO3 contains the ClO3 ion. P4S3 is an unusual molecule with the skeletal structure. (a) Write Lewis structures for P4S3 and the ClO3 ion. (b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species. (c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species. (d) Determine the oxidation states and formal charge of the atoms in P4S3 and the ClO3 ion.arrow_forwardIndicate which orbitals overlap to form the s bonds in each compound.a. BeBr2b. HgCl2c. ICNarrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forward
- Draw the geometric structures of all the molecules. Write all bond angles and write the kind of geometry observed (linear/trigonal planar/tetrahedral) CH₃CH₃ = CH₃COH = CH₃OH = CH₂CH₂ =arrow_forward32W.) A pi bond is formed by the parallel overlap of s orbitalsX.) Since bromine is from Group VIIA, it needs 7 electrons to complete its octetY.) The bond angle between methane's bond pairs is 109.5⁰Z.) The orbital of f with a shape of y(3x²-y²) contains 14 electronsA.) If all 4 statements are trueB.) If 3 of the 4 statements are trueC.) If 2 of the 4 statements are trueD.) If only 1 of the 4 statements is trueE.) If none of the 4 statements is truearrow_forwardPredict all bond angles in each compound. a. CH3Cl b. NH2OH c. CH2=NCH3 d. HC≡CCH2OHarrow_forward
- A. The overlap of atomic orbitals leads to the formation of bonds. Explain what kind and how many orbitals are used to build the hybrid orbitals of a carbon atom in ethylene to give rise to the three sigma bonds. Also, what happens to the orbital(s) not used in the hybridization process? B. What is the relative energy of the hybrid orbitals used by carbon of ethylene in bonding with respect to the original atomic orbitals of carbon in a ground state?arrow_forwardWhat is the hybridization of the N atom in this molecule? What are the hybridizations of the other 4 atoms indicated? ............................ H-G-C-ċ-ċ-ö-H H H :N-H 2 E→:ö: HHO: A Harrow_forwardCH3+ and CH3− are two highly reactive carbon species. a. What is the predicted hybridization and geometry around each carbon atom? b.Two electrostatic potential plots are drawn for these species. Which ion corresponds to which diagram and why?arrow_forward
- 1. Complete the following table. Molecular Formula Atom a b C C₂H4O 2. Use the Lewis structure below to complete the following table. d H b Lewis Structure H a H H-C-C C-H H-C-O: H Electron Geometry Line-Angle Structure H H :O: H C ||||-- H H C CC H d Hybridization to 3. Identify the functional groups in the Lewis structure from question #2. Approx. Bond Anglearrow_forwarddescribe the hybridization, bonding and geometry in a molecule of benzene. Explain the stability in terms of two resonance structures.arrow_forwardWhich of the following correctly describes a pi bond according to valence bond theory? Select one: O A. A pi bond is formed between adjacent atoms when two p orbitals, co- linear with the bond axis, meet "head on". OB. A pi bond is formed when an s orbital overlaps with a hybrid orbital. OC. A pi bond is formed between adjacent atoms when hybrid orbitals, co- linear with the bond axis, overlap. OD. A pi bond is formed when two adjacent, parallel p orbitals overlap along their sides.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY