
Principles of General Chemistry
3rd Edition
ISBN: 9780073402697
Author: SILBERBERG, Martin S.
Publisher: McGraw-Hill College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.86P
Containers A, B, and C are attached by closed stopcocks of negligible volume.
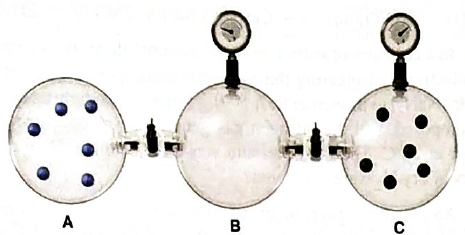
If each particle shown in the picture represents 106 particles,
(a) How many blue particles and black particles are in B after the stopcocks are opened and the system reaches equilibrium?
(b) How many blue particles and black particles are in A after the stopcocks are opened and the system reaches equilibrium?
(c) If the pressure in C,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 5 Solutions
Principles of General Chemistry
Ch. 5 - Prob. 5.1PCh. 5 - Prob. 5.2PCh. 5 - Prob. 5.3PCh. 5 - Prob. 5.4PCh. 5 - Prob. 5.5PCh. 5 - Prob. 5.6PCh. 5 - Prob. 5.7PCh. 5 - Prob. 5.8PCh. 5 - The gravitational force exerted by Earth on an...Ch. 5 - Prob. 5.10P
Ch. 5 - Prob. 5.11PCh. 5 - Prob. 5.12PCh. 5 - Each of the following processes caused the gas...Ch. 5 - What is the effect of the following on the volume...Ch. 5 - What is the effect of the following on the volume...Ch. 5 - Prob. 5.16PCh. 5 - Prob. 5.17PCh. 5 - Prob. 5.18PCh. 5 - Prob. 5.19PCh. 5 - Prob. 5.20PCh. 5 - If 1.4710-3mol of argon occupies a 75.0-mL...Ch. 5 - Prob. 5.22PCh. 5 - A 75.0-g sample of dinitrogen monoxide is confined...Ch. 5 - Prob. 5.24PCh. 5 - Prob. 5.25PCh. 5 - Prob. 5.26PCh. 5 - Prob. 5.27PCh. 5 - Prob. 5.28PCh. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - Prob. 5.31PCh. 5 - Prob. 5.32PCh. 5 - The density of a noble gas is 2.71g/L at 3.00 atm...Ch. 5 - Prob. 5.34PCh. 5 - When an evacuated 63.8-mL glass bulb is tilled...Ch. 5 - After 0.600 L of Ar at 1.20 atm and 227oC is mixed...Ch. 5 - A 355-mL container holds 0.146 g of Ne and an...Ch. 5 - How many grams of phosphorus react with 35.5 L of...Ch. 5 - Prob. 5.39PCh. 5 - Prob. 5.40PCh. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - How many liters of hydrogen gas are collected over...Ch. 5 - Prob. 5.44PCh. 5 - Prob. 5.45PCh. 5 - Prob. 5.46PCh. 5 - Prob. 5.47PCh. 5 - Prob. 5.48PCh. 5 - Prob. 5.49PCh. 5 - Prob. 5.50PCh. 5 - Prob. 5.51PCh. 5 - Prob. 5.52PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - Prob. 5.55PCh. 5 - Prob. 5.56PCh. 5 - Prob. 5.57PCh. 5 - The graph below shows the distribution of...Ch. 5 - Prob. 5.59PCh. 5 - Prob. 5.60PCh. 5 - White phosphorus melts and then vaporizes at high...Ch. 5 - Helium (He) is the lightest noble gas component of...Ch. 5 - Prob. 5.63PCh. 5 - Prob. 5.64PCh. 5 - Prob. 5.65PCh. 5 - Prob. 5.66PCh. 5 - Does SF6(boilingpoint=16oCat1atm) behave more...Ch. 5 - Hemoglobin is the protein that transports O2...Ch. 5 - A baker uses sodium hydrogen carbonate (baking...Ch. 5 - Prob. 5.70PCh. 5 - Prob. 5.71PCh. 5 - Prob. 5.72PCh. 5 - Prob. 5.73PCh. 5 - Prob. 5.74PCh. 5 - Prob. 5.75PCh. 5 - Prob. 5.76PCh. 5 - Prob. 5.77PCh. 5 - Prob. 5.78PCh. 5 - Aluminum chloride is easily vaporized above 180C....Ch. 5 - An atmospheric chemist studying the pollutant SO2...Ch. 5 - The thermal decomposition of ethylene occurs...Ch. 5 - Prob. 5.82PCh. 5 - Analysis of a newly discovered gaseous...Ch. 5 - Prob. 5.84PCh. 5 - Prob. 5.85PCh. 5 - Containers A, B, and C are attached by closed...Ch. 5 - Prob. 5.87PCh. 5 - Prob. 5.88PCh. 5 - Prob. 5.89PCh. 5 - Prob. 5.90PCh. 5 - Prob. 5.91PCh. 5 - Prob. 5.92PCh. 5 - To study a key fuel-cell reaction, a chemical...Ch. 5 - Prob. 5.94PCh. 5 - Prob. 5.95PCh. 5 - Prob. 5.96PCh. 5 - Prob. 5.97PCh. 5 - Prob. 5.98PCh. 5 - Prob. 5.99PCh. 5 - In A, the picture shows a cylinder with 0.1 mol of...Ch. 5 - Prob. 5.101PCh. 5 - Prob. 5.102PCh. 5 - According to government standards, the 8h...Ch. 5 - One way to prevent emission of the pollutant NO...Ch. 5 - Prob. 5.105PCh. 5 - Prob. 5.106PCh. 5 - Prob. 5.107PCh. 5 - Prob. 5.108P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY