
Concept explainers
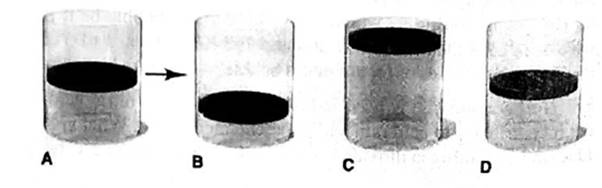
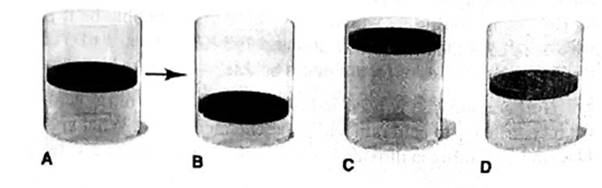
In A, the picture shows a cylinder with 0.1 mol of a gas that behaves ideally. Choose the cylinder (B, C, or D) that correctly represents the volume of the gas after each of the foflowing changes. If none of the cylinders is correct, specify “none.”
(a) P is doubled at fixed n and T
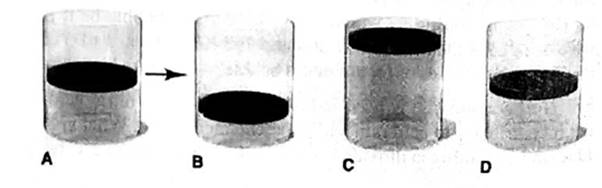
(b) T is reduced Ironi 400 K to 200 K at fixed n and P.
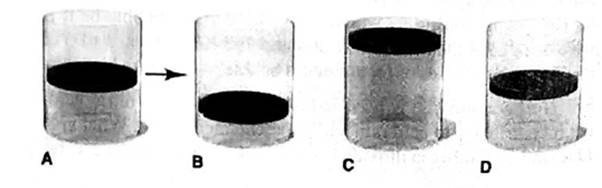
(c) T is increased from 100°C to 200°C at fixed is and P
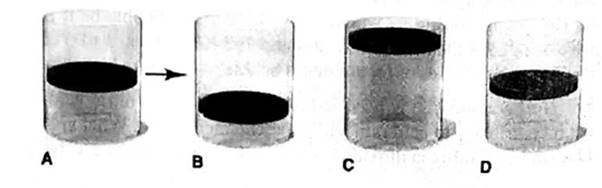
(d) 0.1 mol of gas is added at fixed P and T.
(e) 0.1 mol of gas is added and P is doubled at fixed T.
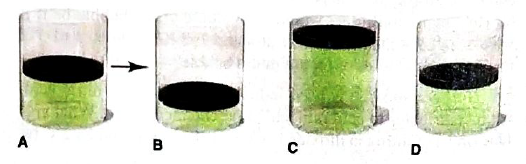
(a)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the pressure of a gas is doubled at fixed number of moles and temperature.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature and number of moles of a gas, the volume of a gas is ½ of the initial volume when the pressure of a gas is doubled.
Thus, it is represents by Cylinder B.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when the pressure is doubled at constant number of moles and temperature.
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature and number of moles of a gas, the volume of a gas is ½ of the initial volume when the pressure of a gas is doubled.
Thus, it is represents by Cylinder B.
(b)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the temperature of a gas is reduced from 400 K to 200 K at constant number of moles and pressure.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
The final volume of a gas is ½ times of the initial volume of a gas and it is described by the Cylinder B.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The ideal gas law for two given conditions is:
Where,
Put the values,
Thus, the final volume of a gas is ½ times of the initial volume of a gas and it is described by the Cylinder B.
(c)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when the temperature of a gas is increased from 100
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
The final volume of a gas is 1.26 times of the initial volume of a gas. It is described by “None” cylinder.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The ideal gas law for two given conditions is:
Where,
Convert the value of temperature in degree Celsius in Kelvin.
Initial temperature in K =
Final temperature in K =
Put the values,
Thus, the final volume of a gas is 1.26 times of the initial volume of a gas and it is not described any cylinder.
(d)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when 0.1 mole of a gas is added at constant temperature and pressure.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature and pressure of a gas, the volume of a gas is 2 times of the initial volume when the 0.1 mole of a gas is added.
Thus, it is represents by Cylinder C.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when 0.1 mole of a gas is added at constant temperature and pressure.
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature and pressure of a gas, the volume of a gas is 2 times of the initial volume when the 0.1 mole of a gas is added.
Thus, it is represents by Cylinder C.
(e)
Interpretation:
The cylinder among B, C and D should be chosen that exactly represents the volume of a gas when 0.1 mole of a gas is added and Pressure is doubled at constant temperature.
Concept Introduction:
Boyle's Law gives the relationship between Pressure (P) and Volume (V).
According to Boyle's Law, the volume of gas changes inversely with the pressure of the gas if temperature and amount of a gas are constant.
PV = constant
The pressure of a gas decreases with increase in volume; volume of a gas decreases with increase in pressure.
Charles’s Law gives the relationship between Volume (V) and Temperature (T)
According to Charles’s Law, the volume of gas has direct relationship with temperature of the gas if pressure and amount of a gas are constant.
If the temperature or volume of a gas changes without any change in amount of a gas and pressure, then the final volume and temperature will give the same
Charles’s Law can be written as:
Where, T1 and V1 are the initial temperature and volume.
T2 and V2 are the final temperature and volume.
Avogadro's Law:
At same condition of pressure and temperature, equal volume of gases has same number of moles. In other words, at same temperature and pressure; one mole of a gas has the same volume.
According to Avogadro's Law, at STP, 1 mole of a gas consist of
The mathematical expression is given as:
Amonton's Law:
The pressure of a gas is directly related with the absolute temperature at constant number of moles and volume.
The mathematical expression is given as:
Or,
Answer to Problem 5.100P
At constant temperature of a gas, the volume of a gas is equal to the initial volume when the 0.1 mole of a gas is added and pressure is doubled.
Thus, it is represents by Cylinder D.
Explanation of Solution
Given information:
The cylinders are:
Ideal gas law gives the relation between pressure, volume, number of moles and temperature.
The ideal gas law is:
Where,
P = Pressure
V = Volume
n = Number of moles
R = Universal gas constant (
T = Temperature
The new ideal expression is shown below, when 0.1 mole of a gas is added and Pressure is doubled at constant temperature
Now, the new volume is calculated as:
Thus, new volume is:
Hence, at constant temperature of a gas, the volume of a gas is equal to the initial volume when the 0.1 mole of a gas is added and pressure is doubled.
Thus, it is represents by Cylinder D.
Want to see more full solutions like this?
Chapter 5 Solutions
Principles of General Chemistry
- b. CH3 H3C 'N' H3C CH3 CN Ph 1. OH N 2. H2O2, Pyridinearrow_forwardFor each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forward
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





