
Concept explainers
Scenes A-D represent atomic-scale views of different samples of substances:
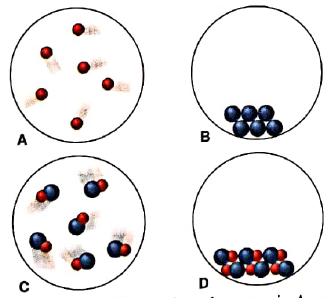
- Under one set of conditions, the substances in A and B mix, and the result is depicted in C. Does this represent a chemical or a physical change?
- Under a second set of conditions, the same substances mix, and the result is depicted in D. Does this represent a chemical or a physical change?
- Under a third set of conditions, the same substances mix, and the result is depicted in D. Does this represent a chemical or a physical change?
- After the change in part(c) has occurred, does the sample have different chemical properties? Physical properties?
a)
Interpretation: Whether combination of A and B to form C is physical or chemical change should be determined.
Concept introduction: Changes can be classified as physical and chemical changes. Physical changes are such changes that allow change of state of matter only but not formation of new substances. Reversal of such changes is possible by physical methods.
Chemical changes allow formation of new and different substances from original substances via chemical reactions. These cannot be reversed back to original state by any method.
Explanation of Solution
Substances that are present in A and B have completely different properties than those formed in C. So when A and B are mixed to form C, it refers to chemical change.
b)
Interpretation: Whether combination of A and B to form D is physical or chemical change should be determined.
Concept introduction:Changes can be classified as physical and chemical changes. Physical changes are such changes that allow change of state of matter only but not formation of new substances. Reversal of such changes is possible by physical methods.
Chemical changes allow formation of new and different substances from original substances via chemical reactions. These cannot be reversed back to original state by any method.
Explanation of Solution
Substances that are present in A and B have completely different properties than those formed in D. So when A and B are mixed to form D, a completely different substance is formed. Therefore combination of A and B to form D is chemical change.
c)
Interpretation: Whether conversion of C to D is physical or chemical change should be determined.
Concept introduction: Changes can be classified as physical and chemical changes. Physical changes are such changes that allow change of state of matter only but not formation of new substances. Reversal of such changes is possible by physical methods.
Chemical changes allow formation of new and different substances from original substances via chemical reactions. These cannot be reversed back to original state by any method.
Explanation of Solution
When sample in C is converted to D, there occurs difference in arrangement of particles only while substances remain same. Therefore it is physical change.
d)
Interpretation: Whether conversion of C to D is physical or chemical change should be determined.
Concept introduction: Changes can be classified as physical and chemical changes. Physical changes are such changes that allow change of state of matter only but not formation of new substances. Reversal of such changes is possible by physical methods.
Chemical changes allow formation of new and different substances from original substances via chemical reactions. These cannot be reversed back to original state by any method.
Explanation of Solution
When sample in C is converted to D, only arrangement of particles is changed while substances remain same. Therefore it is physical change and physical properties are changed in conversion of C to D.
Want to see more full solutions like this?
Chapter 1 Solutions
Principles of General Chemistry
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





