
(a)
Interpretation:
Structural formula of the parent acid and parent alcohol for the given ester molecule has to be drawn.
Concept Introduction:
Esters are prepared by condensation of
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
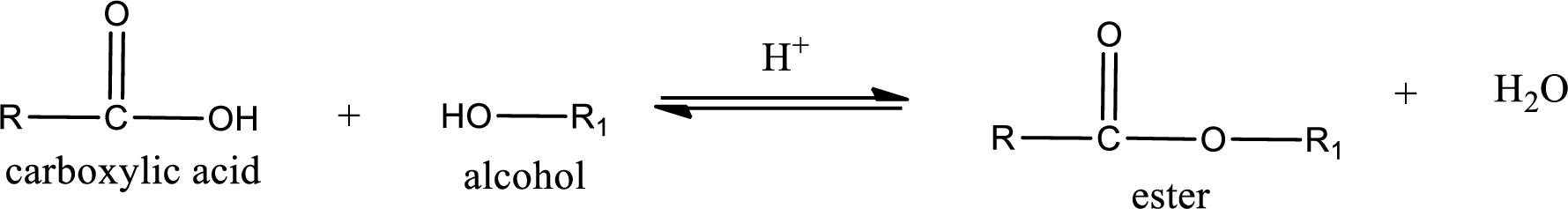
In the above reaction, hydroxyl group is lost from carboxylic acid and hydrogen is lost from alcohol molecule to form water as the byproduct.
(b)
Interpretation:
Structural formula of the parent acid and parent alcohol for the given ester molecule has to be drawn.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
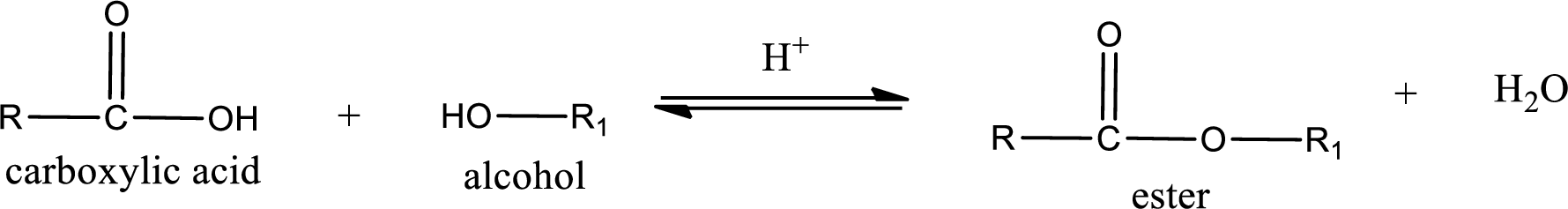
In the above reaction, hydroxyl group is lost from carboxylic acid and hydrogen is lost from alcohol molecule to form water as the byproduct.
(c)
Interpretation:
Structural formula of the parent acid and parent alcohol for the given ester molecule has to be drawn.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
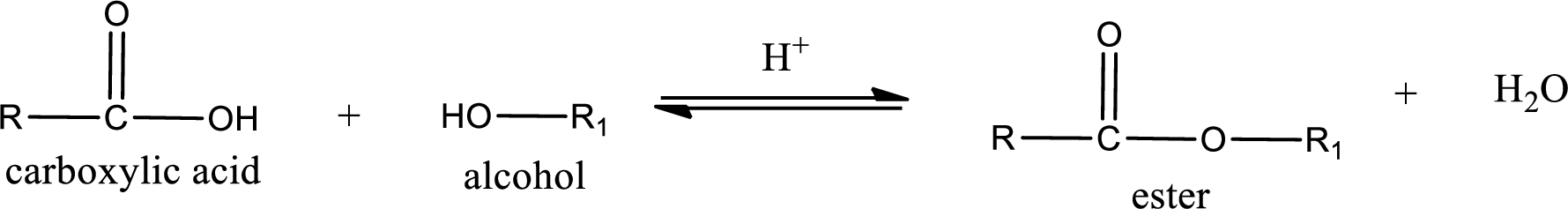
In the above reaction, hydroxyl group is lost from carboxylic acid and hydrogen is lost from alcohol molecule to form water as the byproduct.
(d)
Interpretation:
Structural formula of the parent acid and parent alcohol for the given ester molecule has to be drawn.
Concept Introduction:
Esters are prepared by condensation of carboxylic acid with an alcohol. A molecule of water is lost on this reaction. The reaction that takes place in producing esters is known as esterification reaction.
Esterification reaction is the one in which the carboxylic acid is condensed with an alcohol (or phenol) in presence of strong acid catalyst to produce ester. The general reaction scheme can be given as,
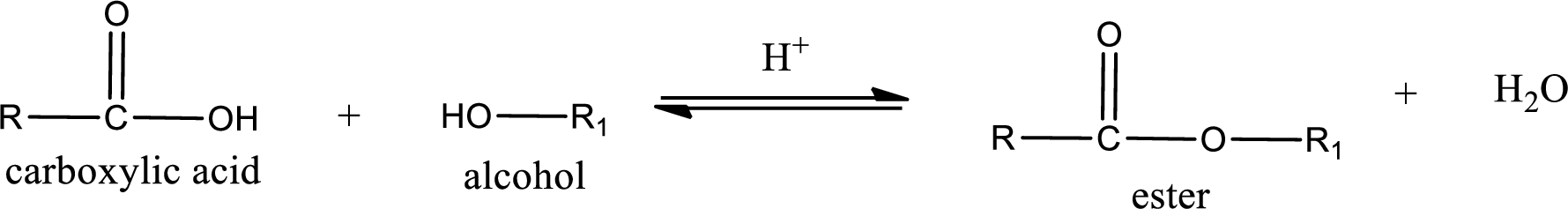
In the above reaction, hydroxyl group is lost from carboxylic acid and hydrogen is lost from alcohol molecule to form water as the byproduct.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic And Biological Chemistry
- .. Name each of the following compounds by IUPAC rules. [three Only] A. 0 B. C. Cl NH₂ OCH N CH3 NHCH2CH3 D. CH O₁₂N NH₂arrow_forwardDraw the structure of the aldol self condensation product for each of the following compounds if a compound does not under go aldol self condensation explain whyarrow_forwardIndicate how the addition of a nucleophile :NuH2 to a ketone R-CO-R occurs.arrow_forward
- Indicate the compound that gives us a primary carbocation and that gives us a secondary carbocation: R-CHO and R-CO-Rarrow_forwardDraw a stepwise mechanism for the following reaction. OHarrow_forwardHelp with annotating the labeled peaks in the 'H NMR (solvent CDCls) spectra and 'H NMR (solvent Acetone-D6) spectra Also help with Calculating the keto-enol tautomerization Ka constant for the product in both solvents.Two solvents and two different Kaarrow_forward
- Draw a Haworth projection of a common cyclic form of this monosaccharide CH₂OH HO H HO H H OH CH₂OHarrow_forwardCan you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forward
- Select/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forwardCan u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





