
(a)
Interpretation:
The structure of thioester formed when reaction takes place between
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
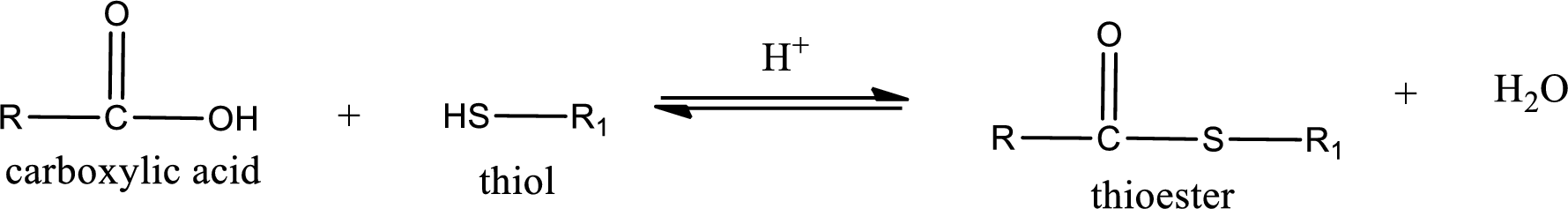
(b)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
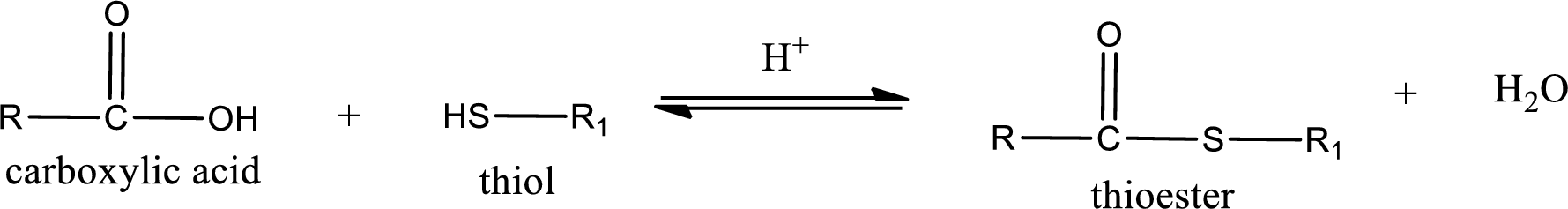
(c)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
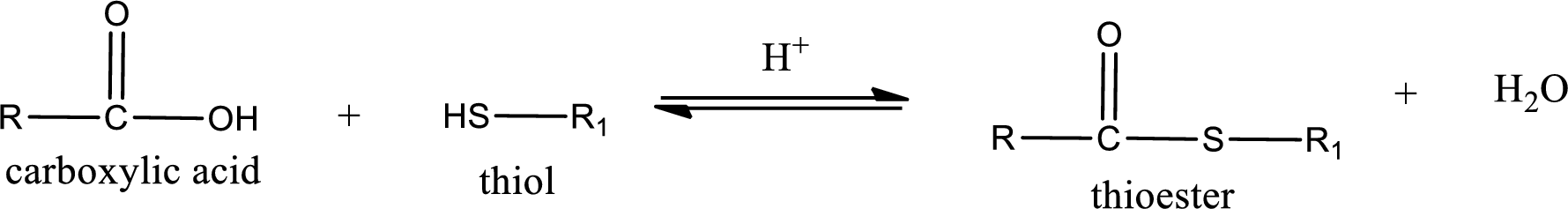
(d)
Interpretation:
The structure of thioester formed when reaction takes place between carboxylic acid given and the thiol given has to be drawn.
Concept Introduction:
Thioesters are prepared by condensation of carboxylic acid with a thiol. A molecule of water is lost on this reaction. The reaction that takes place in producing thioesters is known as thioesterification reaction.
Thioesterification reaction is the one in which the carboxylic acid is condensed with a thiol in presence of strong acid catalyst to produce thioester. The general reaction scheme can be given as,
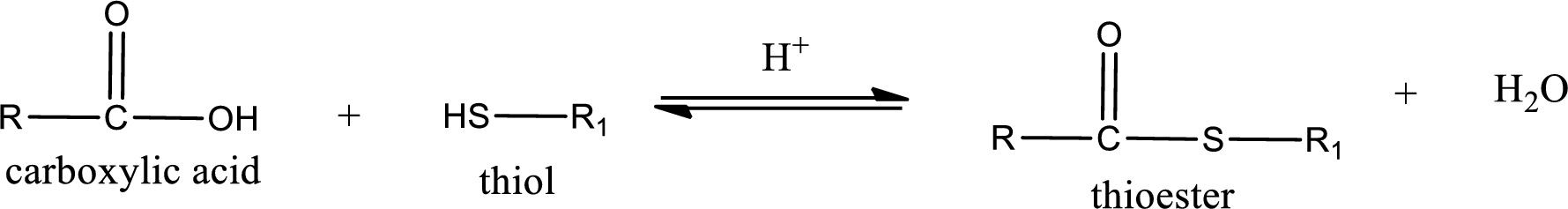
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic And Biological Chemistry
- Please correct answer and don't use hand ratingarrow_forwardNonearrow_forwardYou have started a patient on a new drug. Each dose introduces 40 pg/mL of drug after redistribution and prior to elimination. This drug is administered at 24 h intervals and has a half life of 24 h. What will the concentration of drug be after each of the first six doses? Show your work a. What is the concentration after the fourth dose? in pg/mL b. What is the concentration after the fifth dose? in pg/mL c. What is the concentration after the sixth dose? in pg/mLarrow_forward
- McLafferty Rearrangement: Label alpha (), beta (), and gamma () on the molecule. Draw mechanismarrows to describe the process of the rearrangement. What functional group is lost during the rearrangement? What new functional group is made from the ketone/aldehyde you started with? What stabilizing chemical theory causes (allows) rearrangement to happen?arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raitingarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


