
Concept explainers
Assign an IUPAC name to each of the following
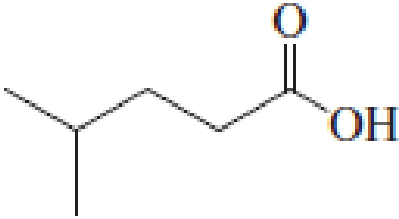
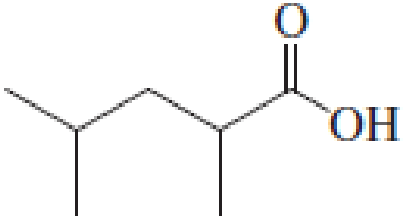
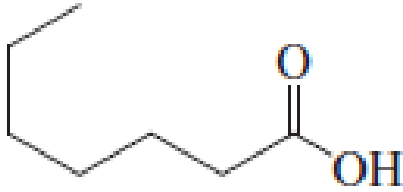
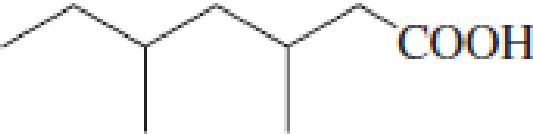
(a)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
Answer to Problem 5.16EP
IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
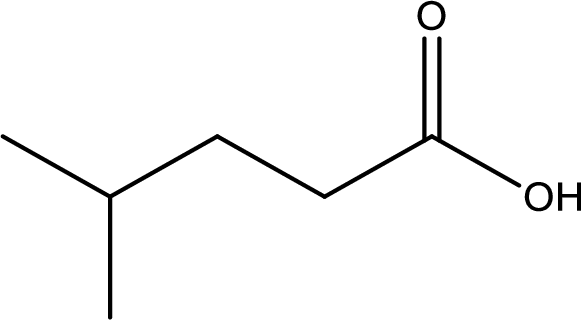
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a five carbon chain. Hence, the parent alkane is pentane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as pentanoic acid.
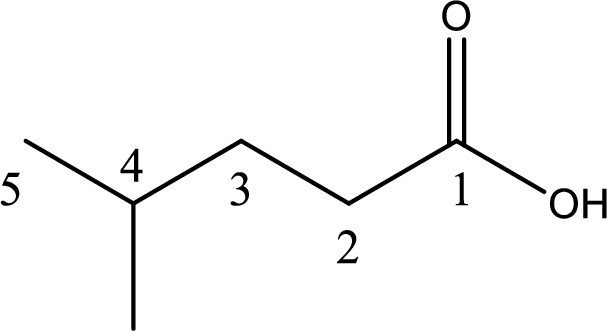
Looking for substituents it is found that there is a methyl group present on fourth carbon atom. Hence, the IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
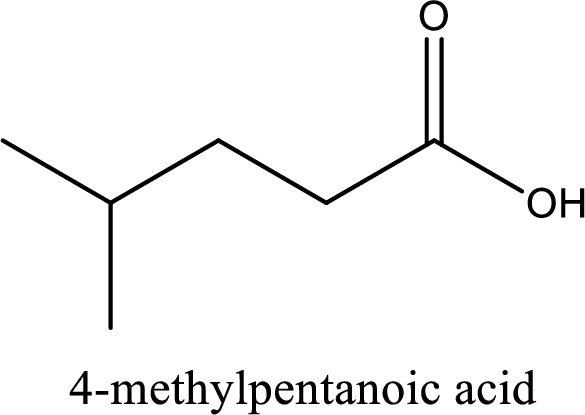
The IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
IUPAC name of the given carboxylic acid is found out.
(b)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for carboxylic acid, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
Answer to Problem 5.16EP
IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
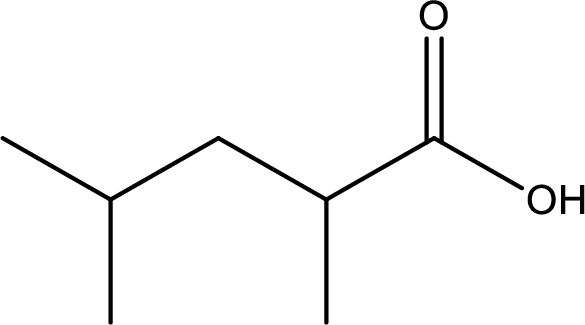
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a five carbon chain. Hence, the parent alkane is pentane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as pentanoic acid.
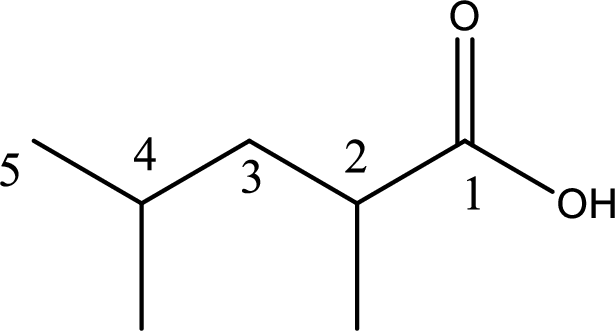
Looking for substituents it is found that there are two methyl groups present, each on second and fourth carbon atom. In the IUPAC name prefix “-di” has to be added before the alkyl name. Hence, the IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
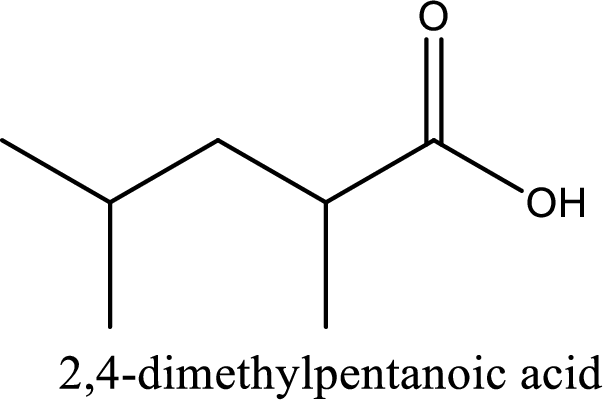
The IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
IUPAC name of the given carboxylic acid is found out.
(c)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
Answer to Problem 5.16EP
IUPAC name of the given carboxylic acid is heptanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
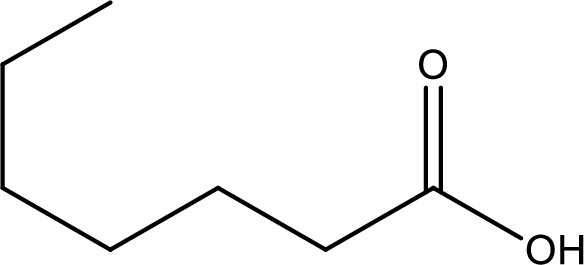
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a seven carbon chain. Hence, the parent alkane is heptane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as heptanoic acid.
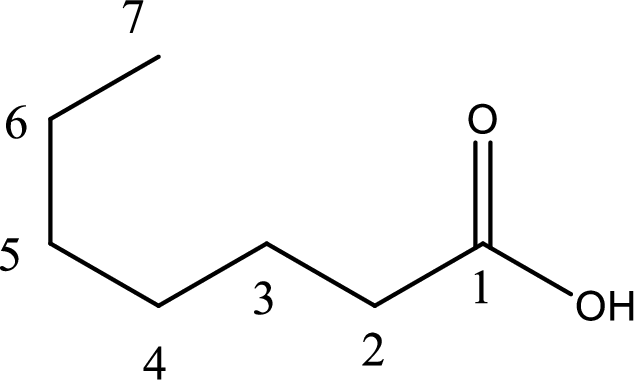
Looking for substituents it is found that there are no substituents present on the carbon chain. Hence, the IUPAC name of the given carboxylic acid is heptanoic acid.
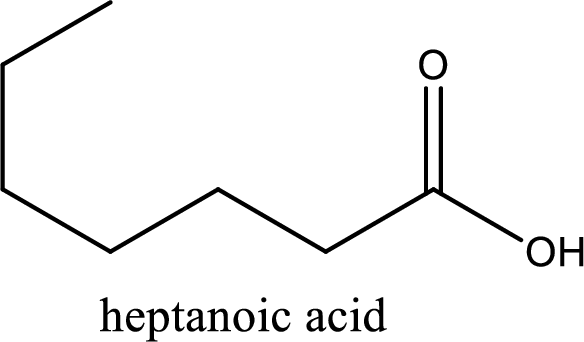
The IUPAC name of the given carboxylic acid is heptanoic acid.
IUPAC name of the given carboxylic acid is found out.
(d)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
Answer to Problem 5.16EP
IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
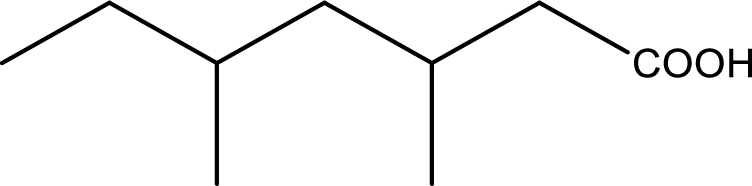
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a seven carbon chain. Hence, the parent alkane is heptane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as heptanoic acid.
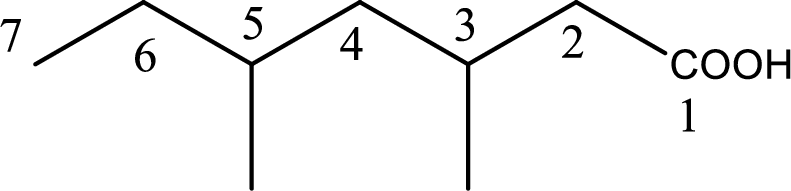
Looking for substituents it is found that there are two methyl groups present as substituents, each on third carbon atom and fifth carbon atom. Hence, the IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
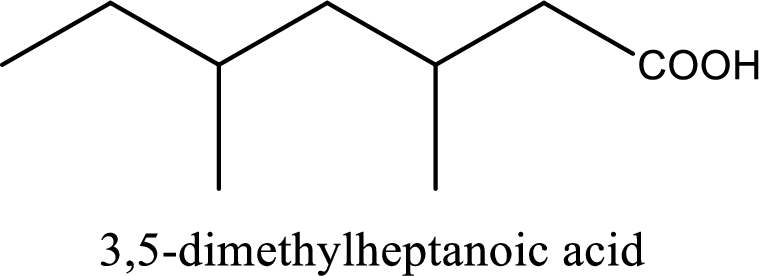
The IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
IUPAC name of the given carboxylic acid is found out.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic And Biological Chemistry
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

