
Concept explainers
(a)
Interpretation:
The given compound is a carbonyl compound or acyl compound has to be classified.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
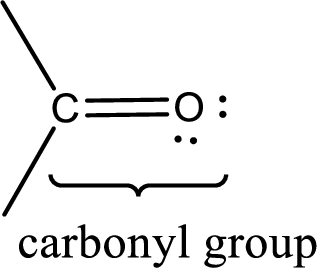
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
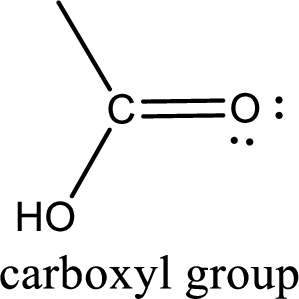
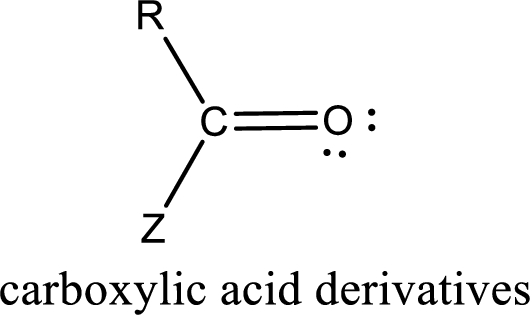
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound.
(b)
Interpretation:
The given compound is a carbonyl compound or acyl compound has to be classified.
Concept Introduction:
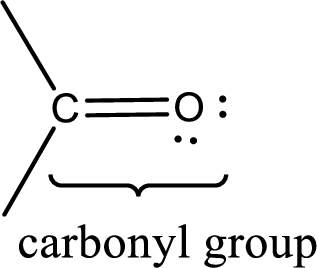
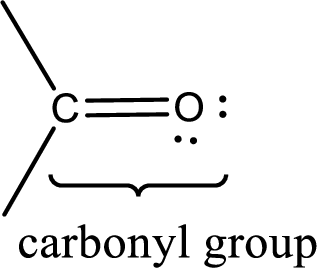
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
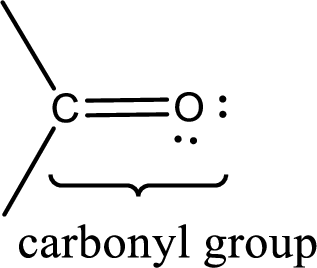
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
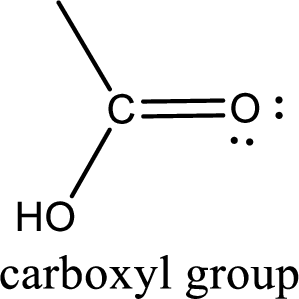
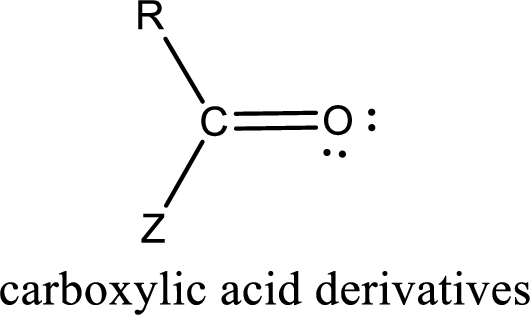
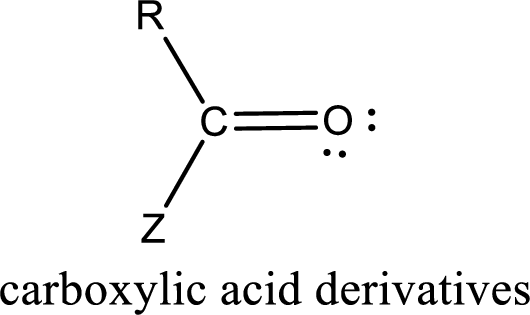
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound.
(c)
Interpretation:
The given compound is a carbonyl compound or acyl compound has to be classified.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
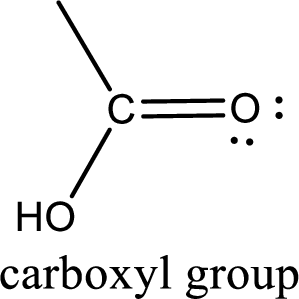
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
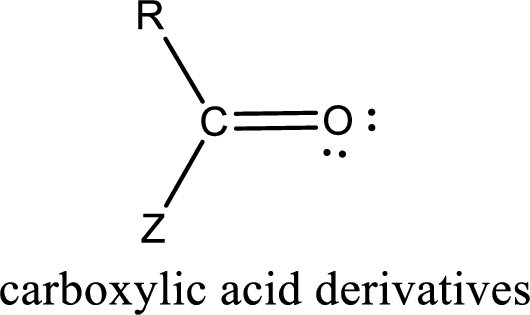
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound.
(d)
Interpretation:
The given compound is a carbonyl compound or acyl compound has to be classified.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
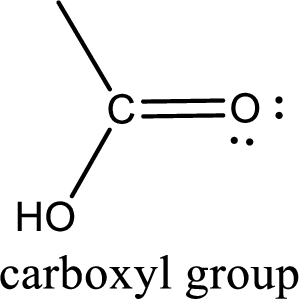
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic And Biological Chemistry
- Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forwardIndicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forward
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



