
(a)
Interpretation:
Chemical equation for the conversion of given
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
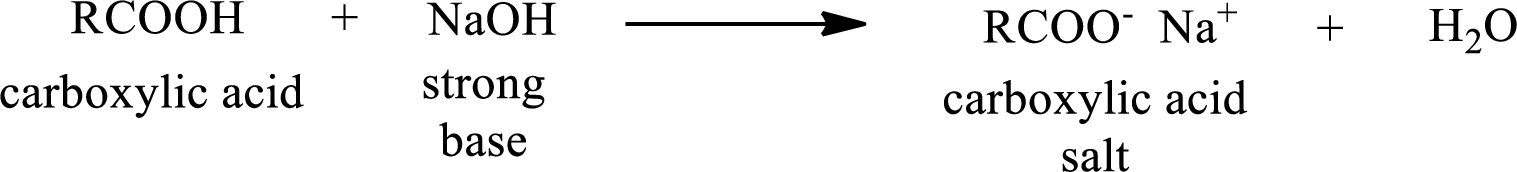
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
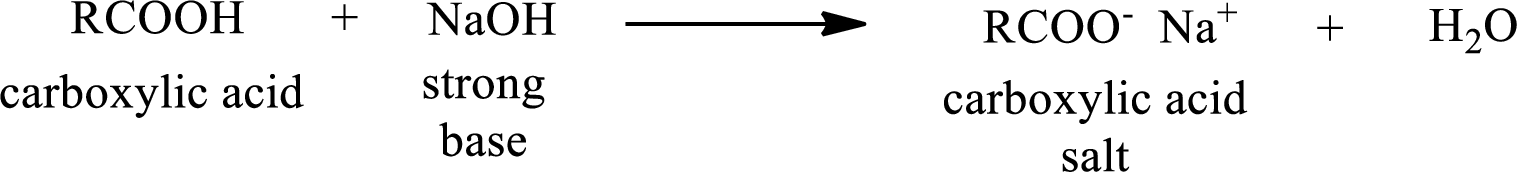
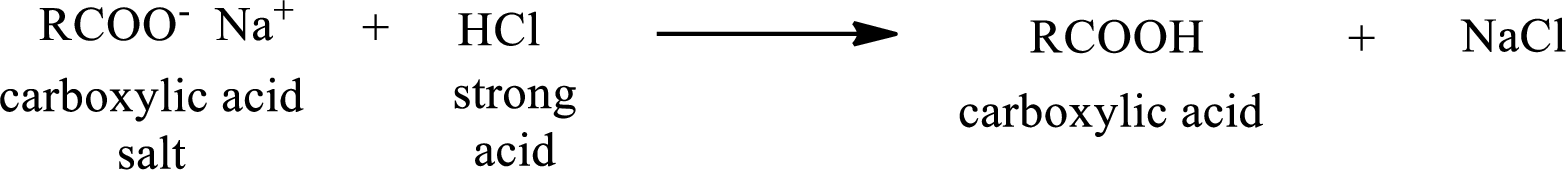
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
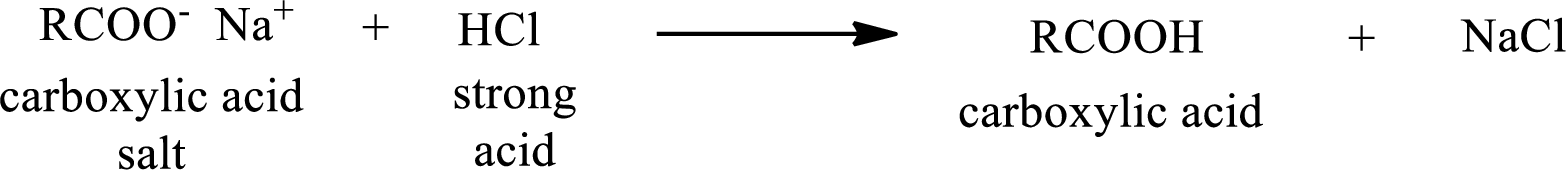
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(a)
Explanation of Solution
Given carboxylic acid salt is sodium butanoate. The structure of sodium butanoate can be given as shown below,
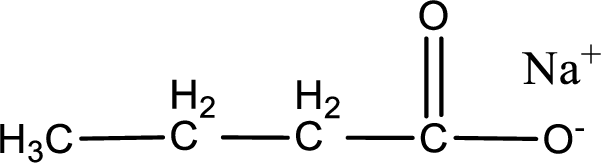
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
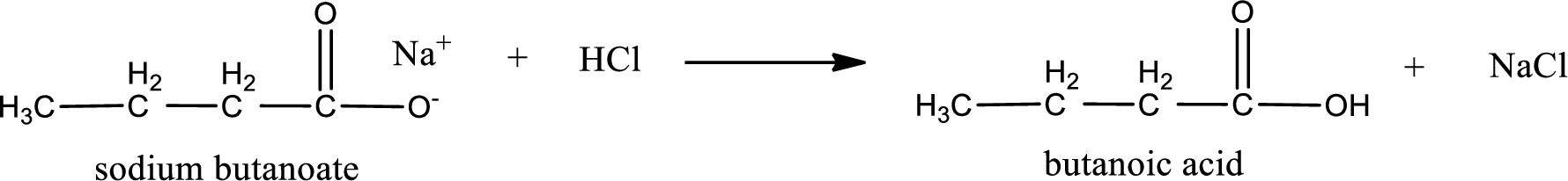
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(b)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
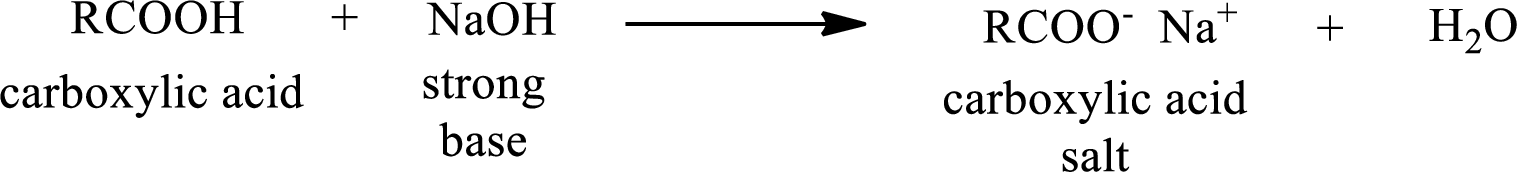
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
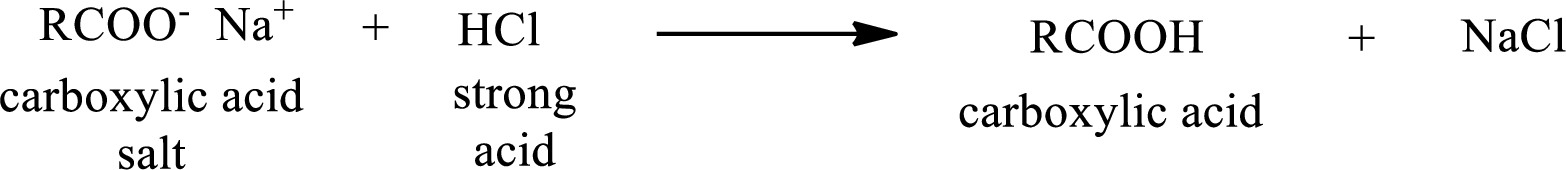
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(b)
Explanation of Solution
Given carboxylic acid salt is potassium oxalate. The structure of potassium oxalate can be given as shown below,
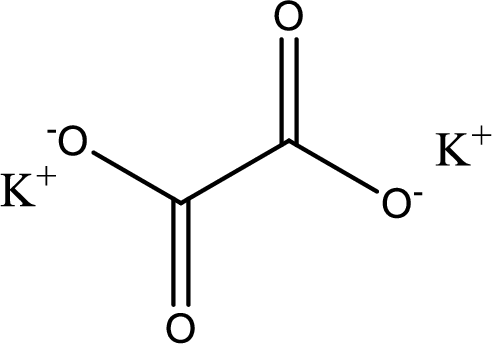
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
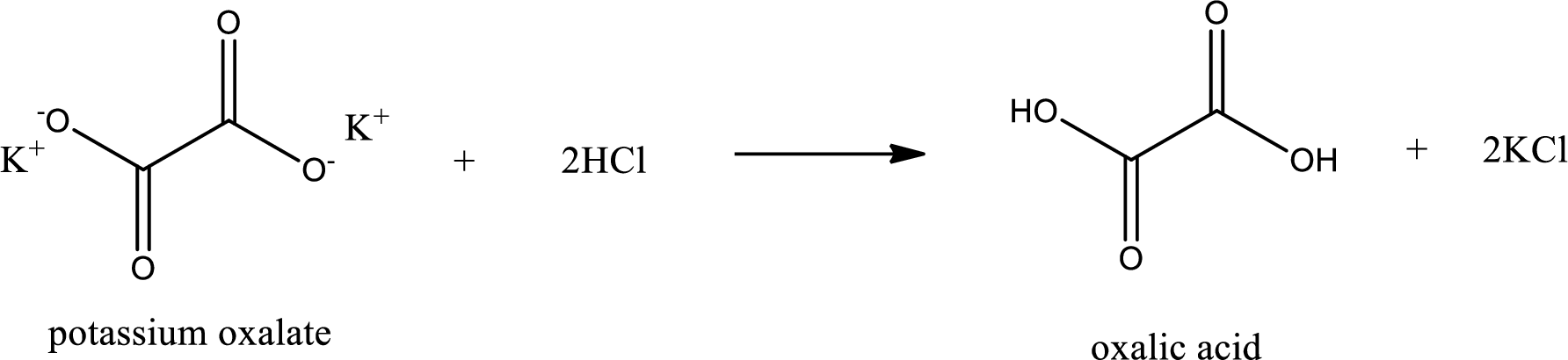
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(c)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(c)
Explanation of Solution
Given carboxylic acid salt is calcium malonate. The structure of calcium malonate can be given as shown below,
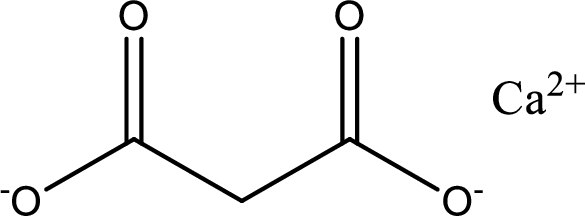
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
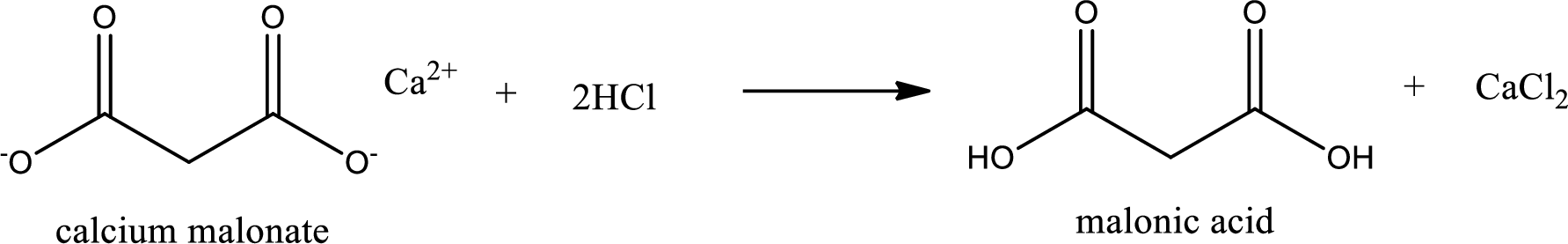
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
(d)
Interpretation:
Chemical equation for the conversion of given carboxylic acid salt to its parent carboxylic acid using
Concept Introduction:
The name of the carboxylic acid itself implies that it is acidic. Addition of carboxylic acid to water results in ionization. Hydrogen ion transfer occurs from carboxylic acid to water and hydronium ion is formed. Carboxylate ion is also formed due to the loss of hydrogen ion from carboxylic acid.
Carboxylate ion is the negative ion which is formed when one or more acidic protons are lost from carboxylic acid. Similar to carboxylic acid it reacts with strong base to form carboxylic acid salt and water.
Carboxylic acid salts when treated with a strong acid produces carboxylic acid as the product.
Carboxylic acid forms carboxylic acid salt by reacting with a strong base. The general reaction scheme for the formation of carboxylic acid salt is given as shown below,
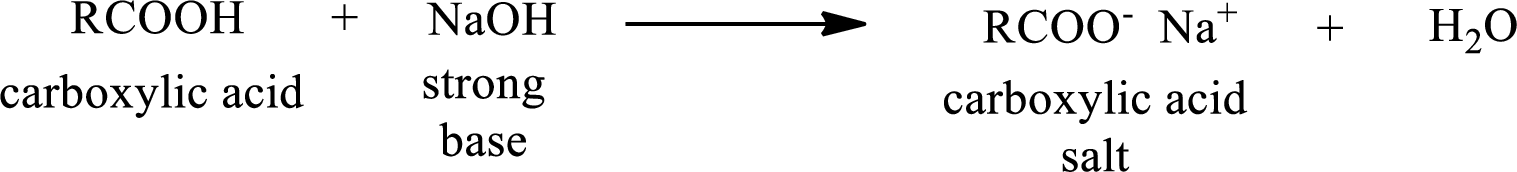
The reverse of the above reaction is conversion of carboxylic acid salt to carboxylic acid. This is accomplished by using strong acid. The scheme is shown below,
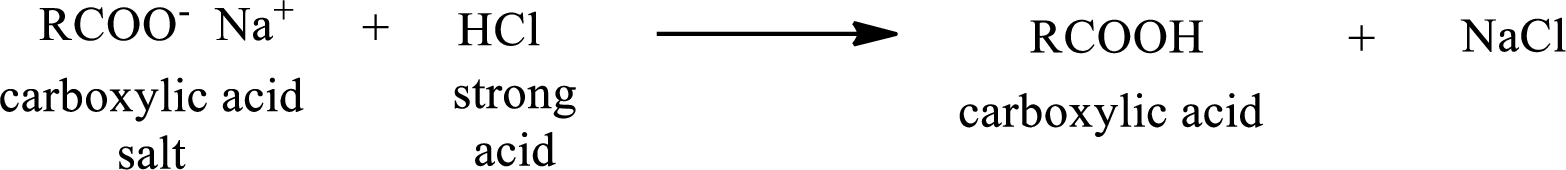
From the above chemical equation it is found that the carboxylic acid salt reacts with strong acid to form carboxylic acid.
(d)
Explanation of Solution
Given carboxylic acid salt is sodium benzoate. The structure of sodium benzoate can be given as shown below,
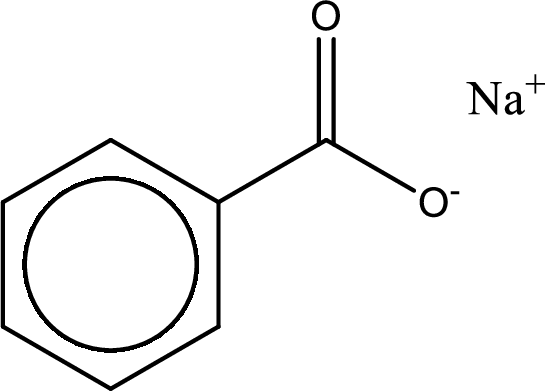
Carboxylic acid sat is converted to carboxylic acid by reaction with strong acid. In the problem statement it is given that the strong acid is
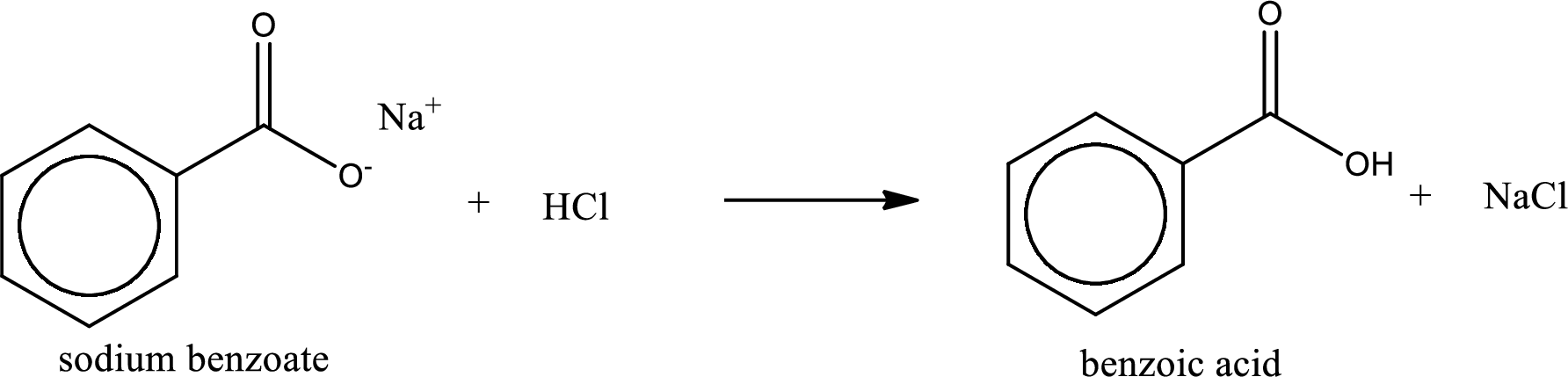
Chemical equation for the conversion of given carboxylic acid salt to carboxylic acid using hydrochloric acid is written.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic And Biological Chemistry
- Part 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- The electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forward
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





