
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 35P
Designate the (R) or (S) configuration at each chirality center in the following molecules.
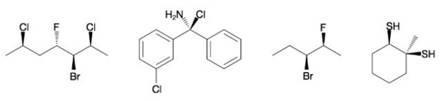
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Vnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling
point, choose 2 next to the substance with the next highest boiling point, and so on.
substance
C
D
chemical symbol,
chemical formula
or Lewis structure.
CH,-N-CH,
CH,
H
H 10: H
C-C-H
H H H
Cale
H 10:
H-C-C-N-CH,
Bri
CH,
boiling point
(C)
Сен
(C) B
(Choose
Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!
Q1: Answer the questions for the reaction below:
..!! Br
OH
a) Predict the product(s) of the reaction.
b) Is the substrate optically active? Are the product(s) optically active as a mix?
c) Draw the curved arrow mechanism for the reaction.
d) What happens to the SN1 reaction rate in each of these instances:
1. Change the substrate to
Br
"CI
2. Change the substrate to
3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF
4. Increase the substrate concentration by 3-fold.
Chapter 5 Solutions
Organic Chemistry
Ch. 5 - Prob. 1PPCh. 5 - Prob. 2PPCh. 5 - Prob. 3PPCh. 5 - Prob. 4PPCh. 5 - Prob. 5PPCh. 5 - Prob. 6PPCh. 5 - Prob. 7PPCh. 5 - Practice Problem 5.8 Write three-dimensional...Ch. 5 - Prob. 9PPCh. 5 - Prob. 10PP
Ch. 5 - Practice Problem 5.11 List the substituents in...Ch. 5 - Prob. 12PPCh. 5 - Practice Problem 5.13 Tell whether the two...Ch. 5 - Prob. 14PPCh. 5 - Prob. 15PPCh. 5 - Prob. 16PPCh. 5 - Prob. 17PPCh. 5 - Prob. 18PPCh. 5 - Prob. 19PPCh. 5 - Prob. 20PPCh. 5 - Practice Problem 5.21 The following are formulas...Ch. 5 - Practice Problem 5.22 Write three-dimensional...Ch. 5 - Prob. 23PPCh. 5 - Practice Problem 5.24 Give names chat include (R)...Ch. 5 - Prob. 25PPCh. 5 - Prob. 26PPCh. 5 - Prob. 27PPCh. 5 - Prob. 28PPCh. 5 - Practice Problem 5.29 Write formulas for all of...Ch. 5 - Prob. 30PPCh. 5 - Prob. 31PPCh. 5 - Prob. 32PPCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - 5.35 Designate the (R) or (S) configuration at...Ch. 5 - Prob. 36PCh. 5 - (a) Write the structure of...Ch. 5 - Shown below are Newman projection formulas for...Ch. 5 - 5.39 Write appropriate structural formulas...Ch. 5 - Discuss whether each of the following compounds...Ch. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 - Compound F has the molecular formula C5H8 and is...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - For the following molecule, draw its enantiomer as...Ch. 5 - 5.49 (Use models to solve this...Ch. 5 - 5.50 (Use models to solve this...Ch. 5 - (Use models co solve this problem.) Write...Ch. 5 - 5.52 Tartaric acid was an important compound in...Ch. 5 - Prob. 53PCh. 5 - Prob. 54PCh. 5 - Prob. 55PCh. 5 - Prob. 1LGPCh. 5 - Prob. 2LGPCh. 5 - Prob. 3LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
The ethmoid bone forms which other cranial structures?
Principles of Anatomy and Physiology
Researchers cross a corn plant that is pure - breeding forthe dominant traits colored aleurone (C1), full kerne...
Genetic Analysis: An Integrated Approach (3rd Edition)
a. Which compound has the stretching vibration for its carbonyl group at the highest frequency: acetyl chloride...
Organic Chemistry (8th Edition)
WHAT IF? A chicken has 78 chromosomes in its somatic cells. How many chromosomes did the chicken inherit from ...
Campbell Biology (11th Edition)
Identify two locations where ice sheets are currently found.
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License