
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 21PP
Practice Problem 5.21
The following are formulas for three compounds, written in noneclipsed conformations. In each instance tell which compound (A, 8, or C on the previous page) each formula represents.
(a)
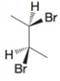
(b)
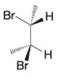
(c)
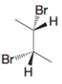
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please explain this in simple terms
K
Most Reactive
Na
(3 pts) Can the metal activity series (shown on the right) or a
standard reduction potential table explain why potassium metal
can be prepared from the reaction of molten KCI and Na metal but
sodium metal is not prepared from the reaction of molten NaCl and
K metal? Show how (not).
Ca
Mg
Al
с
Zn
Fe
Sn
Pb
H
Cu
Ag
Au
Least Reactive
(2 pts) Why is O2 more stable as a diatomic molecule than S2?
Chapter 5 Solutions
Organic Chemistry
Ch. 5 - Prob. 1PPCh. 5 - Prob. 2PPCh. 5 - Prob. 3PPCh. 5 - Prob. 4PPCh. 5 - Prob. 5PPCh. 5 - Prob. 6PPCh. 5 - Prob. 7PPCh. 5 - Practice Problem 5.8 Write three-dimensional...Ch. 5 - Prob. 9PPCh. 5 - Prob. 10PP
Ch. 5 - Practice Problem 5.11 List the substituents in...Ch. 5 - Prob. 12PPCh. 5 - Practice Problem 5.13 Tell whether the two...Ch. 5 - Prob. 14PPCh. 5 - Prob. 15PPCh. 5 - Prob. 16PPCh. 5 - Prob. 17PPCh. 5 - Prob. 18PPCh. 5 - Prob. 19PPCh. 5 - Prob. 20PPCh. 5 - Practice Problem 5.21 The following are formulas...Ch. 5 - Practice Problem 5.22 Write three-dimensional...Ch. 5 - Prob. 23PPCh. 5 - Practice Problem 5.24 Give names chat include (R)...Ch. 5 - Prob. 25PPCh. 5 - Prob. 26PPCh. 5 - Prob. 27PPCh. 5 - Prob. 28PPCh. 5 - Practice Problem 5.29 Write formulas for all of...Ch. 5 - Prob. 30PPCh. 5 - Prob. 31PPCh. 5 - Prob. 32PPCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - 5.35 Designate the (R) or (S) configuration at...Ch. 5 - Prob. 36PCh. 5 - (a) Write the structure of...Ch. 5 - Shown below are Newman projection formulas for...Ch. 5 - 5.39 Write appropriate structural formulas...Ch. 5 - Discuss whether each of the following compounds...Ch. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 - Compound F has the molecular formula C5H8 and is...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - For the following molecule, draw its enantiomer as...Ch. 5 - 5.49 (Use models to solve this...Ch. 5 - 5.50 (Use models to solve this...Ch. 5 - (Use models co solve this problem.) Write...Ch. 5 - 5.52 Tartaric acid was an important compound in...Ch. 5 - Prob. 53PCh. 5 - Prob. 54PCh. 5 - Prob. 55PCh. 5 - Prob. 1LGPCh. 5 - Prob. 2LGPCh. 5 - Prob. 3LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
The glycine cleavage system is a group of four enzymes that together catalyze the following reaction: glycine+T...
Organic Chemistry (8th Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
Modified True/False 9. A giant bacterium that is large enough to be seen without a microscope is Selenomonas.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward
- 1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forwardPlease draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License