
Concept explainers
(a)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of

Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A
(b)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of

Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(c)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
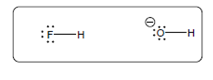
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
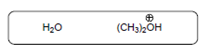
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
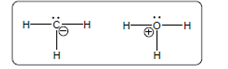
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
(d)
Interpretation:
The acid-base reaction should be described between the following reactant by using curved arrows and each curved arrow having positive or negative should be marked. The value of
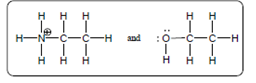
Concept Introduction:
A substance which produces
A substance which produces hydroxide ion when dissolved in aqueous solution is known as a base. These are proton acceptor.
A chemical reaction which takes place between an acid and a base is known as acid-base reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: A Guided Inquiry
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


