
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 18CTQ
Interpretation Introduction
Interpretation: Bond that is broken among reactants and bond that is new among products for below reactions should be labeled.
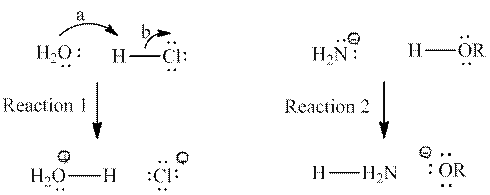
Concept introduction: Reactants are substances that were initially present in
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
In the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?
Indicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.
Chapter 4 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 4 - Prob. 1CTQCh. 4 - Figure 4.1 is a cartoon depiction of liquid water...Ch. 4 - Prob. 3CTQCh. 4 - Prob. 4CTQCh. 4 - In HF , neither H nor F holds a full formal charge...Ch. 4 - Prob. 6CTQCh. 4 - Prob. 7CTQCh. 4 - Prob. 8CTQCh. 4 - Within any one section of Table 4.2, boiling...Ch. 4 - Prob. 10CTQ
Ch. 4 - Prob. 11CTQCh. 4 - Prob. 12CTQCh. 4 - Prob. 13CTQCh. 4 - Prob. 14CTQCh. 4 - Prob. 15CTQCh. 4 - Prob. 16CTQCh. 4 - Prob. 17CTQCh. 4 - Prob. 18CTQCh. 4 - Prob. 19CTQCh. 4 - Prob. 20CTQCh. 4 - Prob. 21CTQCh. 4 - Prob. 22CTQCh. 4 - (E) Label each of the following as strong acid,...Ch. 4 - Prob. 24CTQCh. 4 - Draw the structure of the conjugate base of water....Ch. 4 - Does Cl have a conjugate acid? If so, what is it?...Ch. 4 - Draw the conjugate base of CH4 (methane).Ch. 4 - For the previous four questions, label each...Ch. 4 - Prob. 29CTQCh. 4 - According to the conventions above, what is the...Ch. 4 - Draw an arrow on Figure 4.13 representing Hrxn4 ....Ch. 4 - Prob. 32CTQCh. 4 - Add a + or above each curved arrow in Figure 4.11...Ch. 4 - Prob. 34CTQCh. 4 - Prob. 35CTQCh. 4 - Prob. 36CTQCh. 4 - Prob. 37CTQCh. 4 - Prob. 38CTQCh. 4 - Prob. 39CTQCh. 4 - Prob. 40CTQCh. 4 - Prob. 41CTQCh. 4 - Prob. 42CTQCh. 4 - Prob. 43CTQCh. 4 - Prob. 44CTQCh. 4 - Prob. 45CTQCh. 4 - Prob. 46CTQCh. 4 - For NH3 (ammonia) and H2O (water)... a. Use curved...Ch. 4 - Prob. 48CTQCh. 4 - Prob. 49CTQCh. 4 - Prob. 50CTQCh. 4 - Prob. 51CTQCh. 4 - Prob. 52CTQCh. 4 - Prob. 53CTQCh. 4 - Prob. 1ECh. 4 - Prob. 2ECh. 4 - Prob. 3ECh. 4 - Prob. 4ECh. 4 - Prob. 5ECh. 4 - Prob. 6ECh. 4 - Prob. 7ECh. 4 - Prob. 8ECh. 4 - Propanal (bp 48°C) and propanol (bp 97°C), both...Ch. 4 - Rank the following molecules from lowest to...Ch. 4 - Prob. 12ECh. 4 - For each molecule below, draw the conjugate acid...Ch. 4 - For each structure you drew in the answer to the...Ch. 4 - Mark each of the following statements True or...Ch. 4 - Organic chemistry is a bit like cooking. Later in...Ch. 4 - Prob. 17ECh. 4 - Prob. 18ECh. 4 - Are endothermic reactions favorable or...Ch. 4 - Prob. 20ECh. 4 - Is bond formation endothermic or exothermic? Write...Ch. 4 - Summarize the relationship between pKa and acid...Ch. 4 - Summarize the relationship between pKa and base...Ch. 4 - Prob. 25ECh. 4 - Consider the following bases: a. For each base...Ch. 4 - Prob. 27ECh. 4 - The following are equivalent ways of asking about...Ch. 4 - Prob. 29E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
- pressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning