
Before the element scandium was discovered in 1879, it was known as “eka-boron.” Predict the properties of scandium from averages of the corresponding properties of its neighboring elements in the periodic table. Compare your predictions with the observed values in Appendix F.
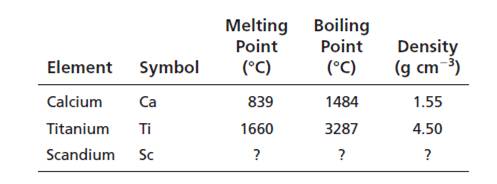
Interpretation:
The properties of scandium should be determined from the averages of the properties of neighbouring elements in the periodic table and predictions should be compared with the observed values in Appendix F.
Concept introduction:
The periodic table contains periods and groups. There are 18 groups and 7 periods in the periodic table. The vertical columns are known as groups and horizontal rows are known as periods.
The numbering of periods is done as 1 to 7 from top to bottom and groups are named as 1A, 2A, 3B to 8B, 1B, 2B, 3A to 8A from left to right where A represents representative elements and B represents transition elements. Elements in the same family have similar chemical and physical properties. In the periodic table, elements are classified as metals, non-metals or metalloids.
Answer to Problem 1P
Calculated values of Scandium are:
Melting point = 1250oC
Boiling point = 2386oC
Density = 3.02 g/cm3
The observed values and calculated values are nearer.
Explanation of Solution
Given information:
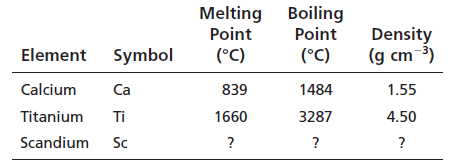
Scandium belongs to group 3 and period 4.The electronic configuration of scandium is:
Melting point is calculated as =
= 1249.5oC
Boiling point is calculated as =
= 2386oC
Density is calculated as =
=
Now, from Appendix F:
Melting point of scandium = 1541oC
Boiling point of scandium = 2386oC
Density of scandium = 2.99 g/cm3
According to the calculated value and observed values, the physical properties are nearer but not exactly the same.
Want to see more full solutions like this?
Chapter 3 Solutions
Principles of Modern Chemistry
- Identify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forwardI need help naming these in IUPACarrow_forward
- H R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forwardhere is my question can u help me please!arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





