
a.
Interpretation: To write the expected products that are obtained by the following reactions.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water,
a.
Answer to Problem 39A
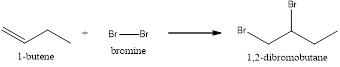
Explanation of Solution
In this reaction, the pi of the alkene and the sigma bond of the alkene will break resulting in the formation of a new bond. This reaction will follow Markonikov’s rule. In the first step, the double bond of the alkene breaks and results in the formation of a carbocation. In the second step, the bond between the halogen breaks and gets attached to the carbocation. Now the halogen will get attached to the carbocation resulting in the formation of 1,2-dibromobutane.
b.
Interpretation: To write the expected products that are obtained by the following reactions.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
b.
Answer to Problem 39A

Explanation of Solution
In this reaction, the pi of the alkene and the sigma bond of the alkene will break resulting in the formation of a new bond. This reaction will follow Markonikov’s rule. In the first step, the double bond of the alkene breaks and results in the formation of carbocation. In the second step, the bond between the halogen breaks and gets attached to the carbocation. Now the halogen will get attached to the carbocation resulting in the formation of 2,3-diiodibutane.
c.
Interpretation: To write the expected products that are obtained by the following reactions.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
c.
Answer to Problem 39A
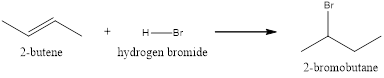
Explanation of Solution
The above reaction is an electrophilic addition reaction which is useful for the preparation of
d.
Interpretation: To write the expected products that are obtained by the following reactions.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
d.
Answer to Problem 39A
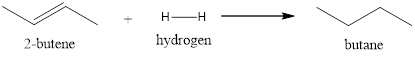
Explanation of Solution
This reaction takes place in the presence of a catalyst. The pi-bond of the alkene will act as a source of electrons for electrophiles. The bond between the hydrogen breaks in the presence of the catalyst resulting in the formation of the metal-hydrogen bond. The surface of the metal absorbs the alkene and helps in adding the other hydrogen resulting in the formation of a saturated compound.
e.
Interpretation: To write the expected products that are obtained by the following reactions.
Concept Introduction: Addition reactions are those in which two or more reactants combine together to form a product. The addition of hydrogen and halogen will follow Markonikov’s rule. In the case of water, alkenes undergo an addition reaction in the presence of a catalyst resulting in the formation of alcohols.
e.
Answer to Problem 39A
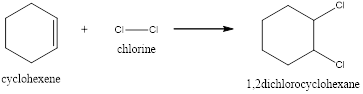
Explanation of Solution
In this reaction, the pi of the alkene and the sigma bond of the alkene will break resulting in the formation of a new bond. In the first step, the double bond of the alkene breaks and results in the formation of a carbocation. In the second step, the bond between the halogen breaks and gets attached to the carbocation. Now the halogen will get attached to the carbocation resulting in the formation of 1,2-dichlorocyclohexane.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Deducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forwardPredict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forward
- Predict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.arrow_forwardcan someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





