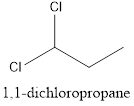
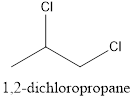
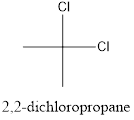
Interpretation: To draw the structural formula and IUPAC name of all possible di-chloropropanes.
Concept Introduction: The structural formula is used to represent the atoms of a chemical compound. It is the way of representing the chemical compound and chemical proportions of an atom using
Answer to Problem 7LC
Isomers are formed when a substitution reaction involving chlorine and propane takes place.
The structural formula along with the IUPAC name is as below:
Explanation of Solution
When a substitution reaction involving chlorine and propane happens, isomers of Di chloropropane are formed. 4 isomers can be formed when this reaction happens, they are:
- 1,1-dichloropropane
- 1,2-dichloropropane
- 1,3-dichloropropane
- 2,2-dichloropropane
4 isomers are formed and all of them are stated above. The isomers are the possible structures that can be formed during the reaction. The
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- If 10 mL of a commercial sodium silicate solution is added, the water required to obtain a 20% solids solution (SiO2+Na2O) is added. Indicate the final grams of Na2SiO3.arrow_forwardPlease help me figure out the mechanism with arrows of the following reactionarrow_forwardOrganic Functional Groups Predicting the reactants or products of acetal hydrolysis termine the structures of the missing organic molecules in the following reaction: H* H* + H₂O Y ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure Explanation Check @2 W Click and drag to start drawing a structure. #4 # 3 LU E % 67 olo 5 66 R T Y & 7 AcGraw Hill LLC. All Rights R Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY