
a.
Interpretation: To determine the products that are obtained by the following reactions.
Concept Introduction: Generally, under normal conditions, bromobenzene will not react with aqueous NaOH because haloarenes are less liable to nucleophilic substitution than
a.
Answer to Problem 36A
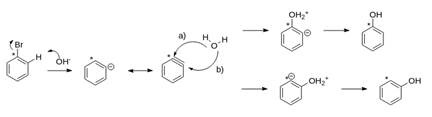
Explanation of Solution
Bromo benzene and sodium hydroxide will not undergo ordinary conditions that are at normal temperature and pressure. This reaction will occur only at high temperatures and pressure that result in the formation of phenol and
b.
Interpretation: To determine the products that are obtained by the following reactions.
Concept Introduction: The given reaction is a substitution reaction. In substitution reactions, one atom or
b.
Answer to Problem 36A
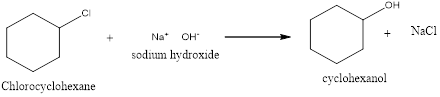
Explanation of Solution
In this reaction, the Chloro-cyclohexane which reacts with sodium hydroxide will form cyclohexanol. The chlorine which is a halogen in this case will be replaced by the hydroxyl group from the sodium hydroxide. This substitution of the group will result in the formation of cyclohexanol and sodium chloride salt.
c.
Interpretation: To determine the products that are obtained by the following reactions.
Concept introduction: The given reaction is a substitution reaction. In substitution reactions, one atom or functional group is replaced with another atom or functional group. In this reaction, the halogen is replaced by the Hydroxyl group.
c.
Answer to Problem 36A
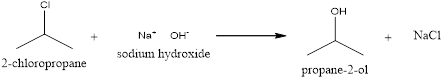
Explanation of Solution
In the above reaction, the chlorine present in the 2-chloropropane is replaced by the hydroxyl group from the sodium hydroxide. This reaction will result in the formation of an alcohol called propane-2-ol along with the sodium chloride salt.
d.
Interpretation: To determine the products that are obtained by the following reactions.
Concept introduction: The given reaction is an electrophilic
d.
Answer to Problem 36A

Explanation of Solution
In the above reaction, bromine is the electrophile. This electrophile will replace the hydrogen of the benzene ring and result in the formation of bromobenzene without any change in the aromaticity of the benzene ring. This reaction is generally used to preserve the aromaticity of the benzene ring.
Chapter 23 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Hello please, draw the main product in the following reactions:arrow_forwardPlease Draw the structure that corresponds to each of the following names: 1-(2-bromopropyl)-3-tert-butyl-6-ethyl-4-methylcyclohexa-1,4-dienearrow_forwardPlease calcula the degrees of unsaturation for the following molecular formula: C6H7NF2. Draw two different compounds with this same molecular formulaarrow_forward
- Which is a bond-line drawing of (CH3)2CHCH2OC(CH3)3 a. b. CL d. d a barrow_forwardWhich of the compounds would have the weakest conjugate base? Post acetylene with a Ka = 1 X 10-25 2-chloroethanol with a Ka = 2 X 10-14 cyclohexanone with a Ka = 5 X 10-16 cyclopentadiene with a Ka = 1 X 10-15arrow_forwardIdentify the meso compound(s). OH OH I only Il only I and II I, II, and III II HO OH OHarrow_forward
- Which compound has the highest boiling point? Q= OH OHarrow_forwardWhat is/are the major product(s) of this reaction? HBr (R)-3-bromo-2,3-dimethylpentane only (S)-3-bromo-2,3-dimethylpentane only a 50:50 mixture of (R) and (S)-3-bromo-2,3-dimethylpentane a 70:30 mixture of (R) and (S)-3-bromo-2,3-dimethylpentanearrow_forwardWhich energy diagram represents a two step reaction where the first step is the rate determining ste E E Reaction Coordinate A Reaction Coordinate C Do NOT Copy or Post OGMU OA OB Ос D E E M Reaction Coordinate B Reaction Coordinate D Aarrow_forward
- Give the best description for the regioselectivity and stereospecificity in the hydroboration-oxidation of an alkene. Markovnikov orientation with syn-addition Markovnikov orientation with anti-addition Anti-Markovnikov orientation with syn-addition Anti-Markovnikov orientation with anti-addition Markovnikov orientation with both syn- and anti-additionarrow_forwardWhich atoms can the negative charge on atom 3 be moved to using resonance theory? 4 6 8 CH 3 2 5 7 only 1, 5, 7 only 2, 4, 6 only 5, 8 only 1 only 2,4arrow_forwardO What is the major product of this reaction? OTS NaOEt OEt OEt ACMUAvousernkarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





