
(a)
Interpretation: The primary carbon needs to be identified.
Concept Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen
- Secondary carbon: When a carbon atom is attached to two carbon atoms and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(a)
Explanation of Solution
In the given molecule,
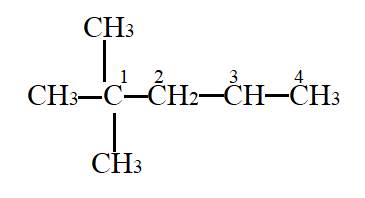
Carbon atom labelled as
(b)
Interpretation- To identify the secondary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(b)
Explanation of Solution
In the given molecule,
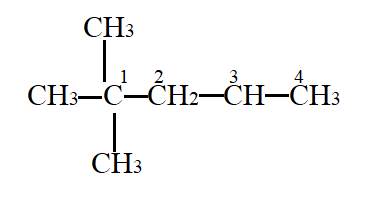
Carbon atom labelled as
(c)
Interpretation- To identify the tertiary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(c)
Explanation of Solution
In the given molecule,
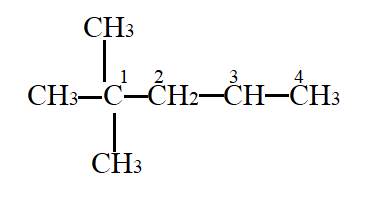
Carbon atom labelled as
(d)
Interpretation- To identify the quaternary carbon.
Concept Introduction-
Carbon atoms can be classified on the basis of carbon attached to them as:
- Primary carbon: When only one carbon atom attached to it. Remaining all the bonds attached to hydrogen.
- Secondary carbon: When carbon atoms is attached to carbon two carbon and remaining two are hydrogen atoms.
- Tertiary carbon: When carbon is attached to 3 carbon atoms and one hydrogen atom.
- Quaternary carbon: When carbon atom is attached to four carbon atoms and no hydrogen atom.
(d)
Explanation of Solution
In the given molecule,
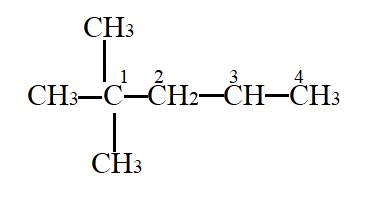
Carbon atom labelled as
Chapter 22 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- how to get limiting reactant and % yield based off this data Compound Mass 6) Volume(mL Ben zaphone-5008 ne Acetic Acid 1. Sam L 2-propanot 8.00 Benzopin- a col 030445 Benzopin a Colone 0.06743 Results Compound Melting Point (°c) Benzopin acol 172°c - 175.8 °c Benzoping to lone 1797-180.9arrow_forwardAssign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forward
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





