
Concept explainers
Interpretation:
The structure of melibiose based on the given information is to be identified.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
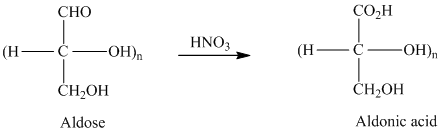
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
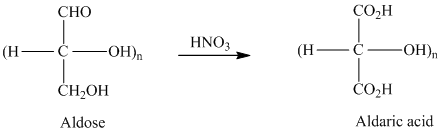
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 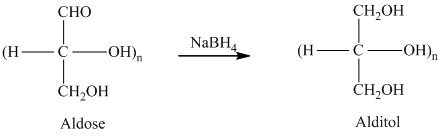
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 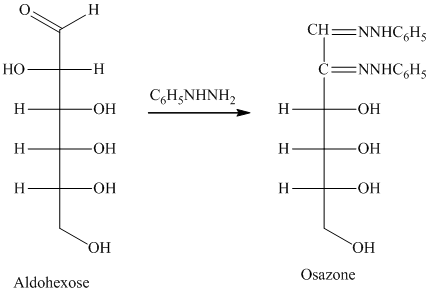
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧 Bromine water is an effective reagent that selectively oxidizes the
舧 Melibiose is a disaccharide carbohydrate with the molecular formula
舧 The aldehyde group of an aldose reacts with three moles of phenylhydrazine to produce phenylosazone at
舧 In aqueous solution, the two anomers and the straight-chain structure of a monosaccharide form an equilibrium mixture between them through the process of mutarotation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- The product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forwardExplain what is the maximum absorbance of in which caffeine absorbs?arrow_forwardExplain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forward
- Protecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forward
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





