
Interpretation:
The products that would be formed on salicin reacting with dilute aqueous HCl is to be determined and the mechanism of reaction for the this reaction is to be outlined.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron-rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
舧 Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
舧 Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
舧 A carbohydrate is a
舧 Salicin is a naturally occurring glycoside and is originally found in the bark of willow trees. It is an effective analgesic for relieving pain. Its structure is given below:
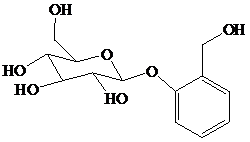
舧 Glycosides are carbohydrate acetals and can be simple or complex. These molecules are stable in basic solutions, but produce sugar and alcohol in acidic solutions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- In a benzene derivative that has -CH2CH3, indicate how it can be substituted by -COOH.arrow_forwardIn a sulfonated derivative of benzene, indicate how -SO3H can be eliminated.arrow_forwardWhat is the equilibrium expression (law of mass action) for the following reaction:CO2 (g) + H2O (l) ⇋ H+ (aq) + HCO3- (aq)arrow_forward
- Indicate the compound resulting from adding NaOH cyclopentane-CH2-CHO.arrow_forwardUse the provided information to calculate Kc for the following reaction at 550 °C: H2(g) + CO2(g) ⇌ CO(g) + H2O(g) Kc = ?CoO(s) + CO(g) ⇌ Co(s) + CO2(g) Kc1 = 490CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc2 = 67arrow_forwardCalculate Kc for the reaction: I2 (g) ⇋ 2 I (g) Kp = 6.26 x 10-22 at 298Karrow_forward
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardTo convert cyclopentane-CH2-CHO to cyclopentane-CH2-CH3, compound A is added, followed by (CH3)3CO-K+, DMS at 100oC. Indicate which compound A is.arrow_forwardIndicate how to obtain the compound 2-Hydroxy-2-phenylacetonitrile from phenylmethanol.arrow_forward
- Indicate the reagent needed to go from cyclopentane-CH2-CHO to cyclopentane-CH2-CH=CH-C6H5.arrow_forwardesc Write the systematic name of each organic molecule: structure CH3 CH3-C=CH2 CH3-CH2-C-CH2-CH3 CH-CH3 CH3 ☐ ☐ ☐ CI-CH-CH=CH2 Explanation Check F1 F2 name 80 F3 F4 F5 F6 A 7 ! 2 # 3 4 % 5 6 & 7 Q W E R Y FT 2025 Mcarrow_forwardTwo reactants X and Z are required to convert the compound CH3-CH2-CH2Br to the compound CH3-CH2-CH=P(C6H5)3. State reactants X and Z.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


