
Concept explainers
Interpretation:
The distinguishing tests for the pair of given compounds are to be determined.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
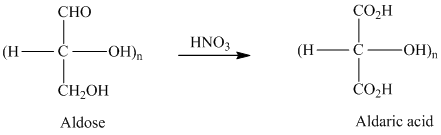
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 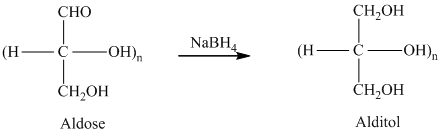
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 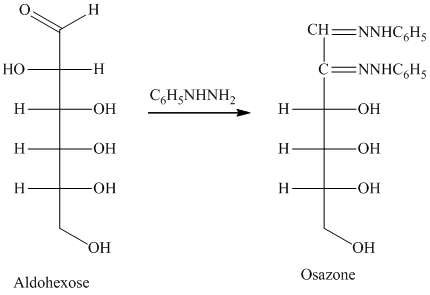
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧. Bromine water is an effective reagent that selectively oxidizes the
舧 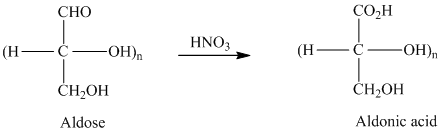
Tollen’s (
舧 Compounds that have plane of symmetry tend to form meso compounds. A meso form arises when the two stereoisomers produce superimposable (achiral) images, and hence, compounds having meso are optically inactive. Chiral (or non-superimposable) molecules are optically active.
舧
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priva ×arrow_forwardPredict the products of this organic reaction: Explanation Check IN NaBH3CN H+ ? Click and drag to start drawing a structure. D 5 C +arrow_forwardPredict the products of this organic reaction: H3O+ + ? • Draw all the reasonable products in the drawing area below. If there are no products, because no reaction will occur, check the box under the drawing area. • Include both major and minor products, if some of the products will be more common than others. • Be sure to use wedge and dash bonds if you need to distinguish between enantiomers. No reaction. Click and drag to start drawing a structure. dmarrow_forward
- Iarrow_forwardDraw the anti-Markovnikov product of the hydration of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for esc esc ☐ Explanation Check F1 1 2 F2 # 3 F3 + $ 14 × 1. BH THE BH3 2. H O NaOH '2 2' Click and drag to start drawing a structure. F4 Q W E R A S D % 905 LL F5 F6 F7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility < & 6 7 27 8 T Y U G H I F8 F9 F10 F11 F12 9 0 J K L P + // command option Z X C V B N M H H rol option commandarrow_forwardAG/F-2° V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: -0.93 +0.38 -0.50 -0.51 -0.06 H3PO4 →H4P206 →H3PO3 →→H3PO₂ → P → PH3 Acidic solution Basic solution -0.28 -0.50 3--1.12 -1.57 -2.05 -0.89 PO HPO H₂PO₂ →P → PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P206 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH P 0.0 -0.5 -1.0- -1.5- -2.0 H.PO, -2.3+ -3 -2 -1 1 2 3 2 H,PO, b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) H,PO 4 S Oxidation stale, Narrow_forward
- 4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward
- 1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 3 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




