
(a)
Interpretation:
The complete, detailed mechanism and the products are to be drawn for the given reaction.
Concept introduction:
Answer to Problem 21.39P
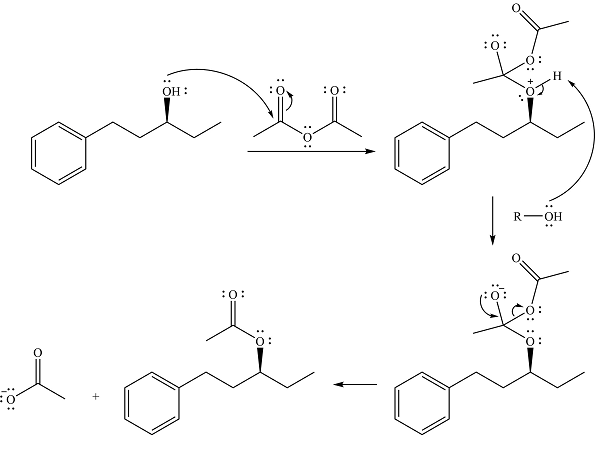
The complete mechanism is
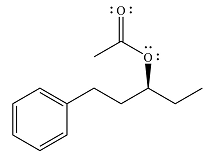
The product of the reaction is
Explanation of Solution
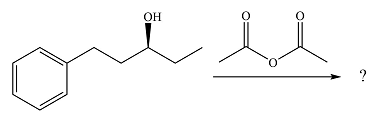
The given reaction is
Alcohol is a weak nucleophile and adds to one of the carbonyl carbons of acetic anhydride to produce a protonated ether linkage. The carbonyl oxygen becomes negatively charged as a result of the
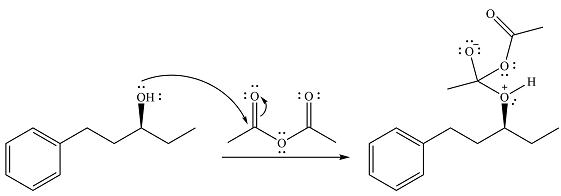
In the next step, the positively charged oxygen is deprotonated by another molecule of the alcohol.
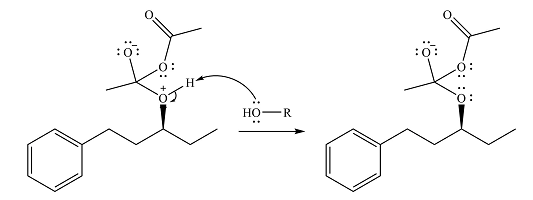
The final step is nucleophilic elimination of acetate anion as a result of the lone pair of the negatively charged oxygen moving to reform the carbonyl group.
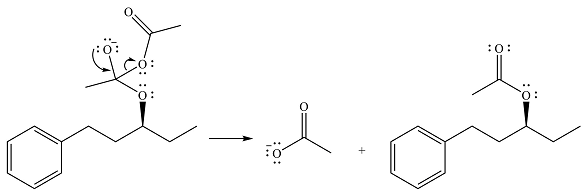
Thus, the complete mechanism of the reaction can be drawn as

The product of the reaction is

The product and mechanism of the given reaction are determined on the basis of nucleophilic addition-elimination mechanism.
(b)
Interpretation:
The complete, detailed mechanism and the products are to be drawn for the given reaction.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 21.39P
The complete mechanism is
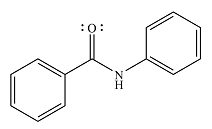
The product of the reaction is
Explanation of Solution
The given reaction is
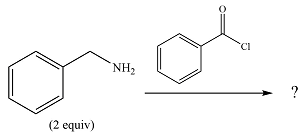
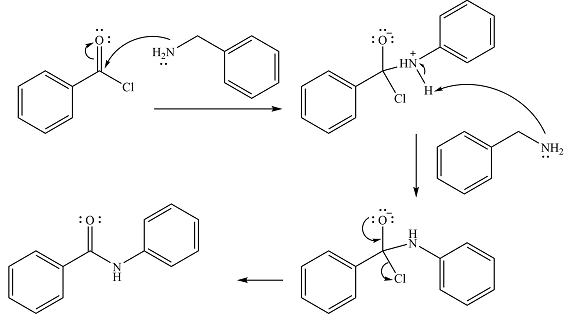
The
In the first step, it acts as a nucleophile and adds to the carbonyl carbon of the acid chloride to produce a protonated amine linkage. The
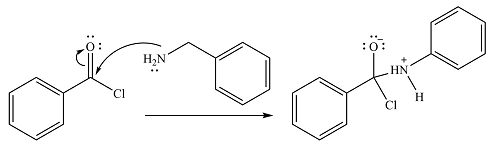
The protonated amine is then deprotonated by a second molecule of the amine.
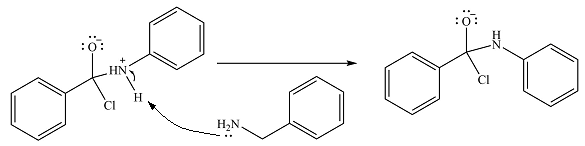
Finally, one lone pair on the negatively charged oxygen will move back to reform the carbonyl group, eliminating the chloride and forming the product.
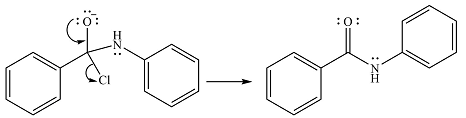
Thus, the complete mechanism can be drawn as

And the product of the reaction will be

The product and mechanism of the given reaction are determined on the basis of nucleophilic addition-elimination mechanism.
(c)
Interpretation:
The complete, detailed mechanism and the products are to be drawn for the given reaction.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 21.39P
The complete mechanism of the reaction is
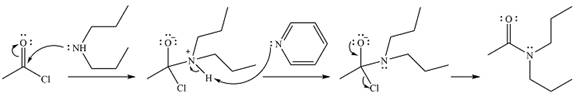
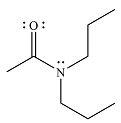
The product of the reaction is
Explanation of Solution
The given reaction is
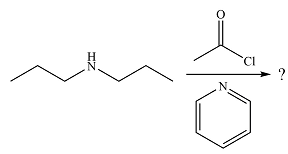
The amine will act as a nucleophile and add to the carbonyl carbon of the acid chloride in the first step to form a protonated amine linkage. The
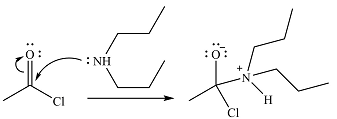
The protonated amine is deprotonated in the second step by the added base, pyridine.
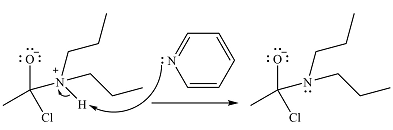
Finally, one lone pair on the negatively charged oxygen moves back to reform the carbonyl group, eliminating the leaving group chloride and forming the final product.
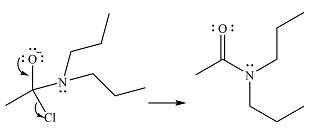
Thus, the complete mechanism can be drawn as

And the product of the reaction will be

The product and mechanism of the given reaction are determined on the basis of nucleophilic addition-elimination mechanism.
(d)
Interpretation:
The complete, detailed mechanism and the products are to be drawn for the given reaction.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 21.39P
The complete mechanism of the reaction is
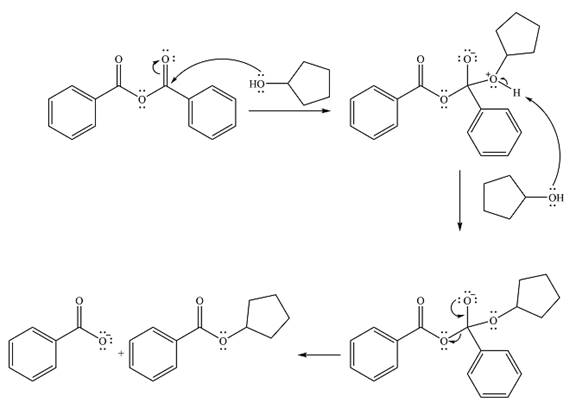
The product of the reaction is
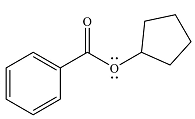
Explanation of Solution
The given reaction is
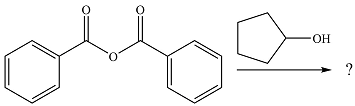
The alcohol is a weak nucleophile. It will add to one of the carbonyl carbons in the anhydride to produce a protonated intermediate.
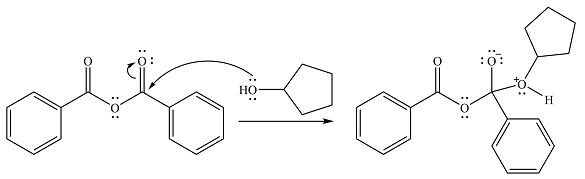
In the seconds step, this intermediate will be deprotonated by a second molecule of the alcohol.
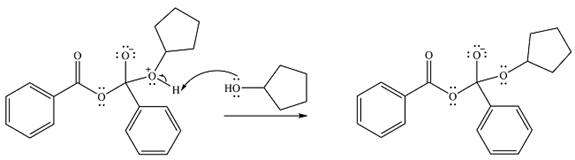
Finally, one lone pair from the negatively charged oxygen will move back to reform the carbonyl, eliminating the leaving group benzoate in the process and forming the product.
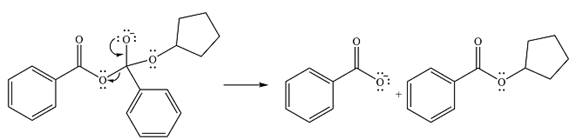
Thus, the complete mechanism can be drawn as

And the product of the reaction will be
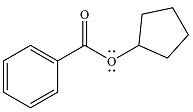
The product and mechanism of the given reaction are determined on the basis of nucleophilic addition-elimination mechanism.
Want to see more full solutions like this?
Chapter 21 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





