
(a)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
The ester is hydrolyzed to the
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
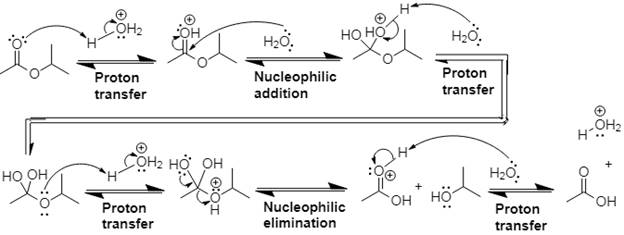
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(b)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid-catalyzed transesterification, and a new ester is formed.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
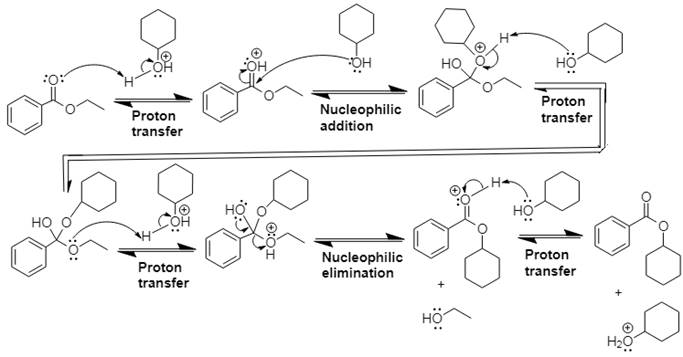
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed transesterification.
(c)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
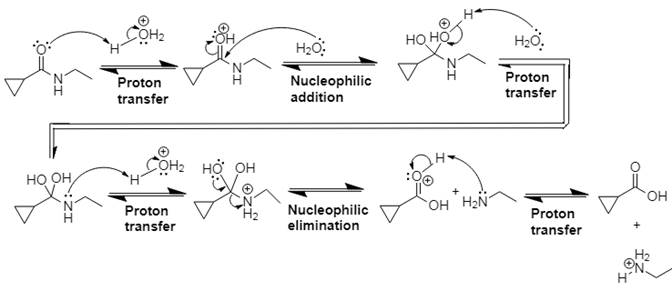
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
(d)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed ester hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of alcohol. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
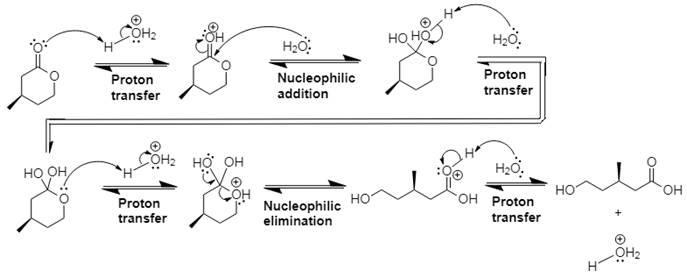
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(e)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is Fischer esterification reaction.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
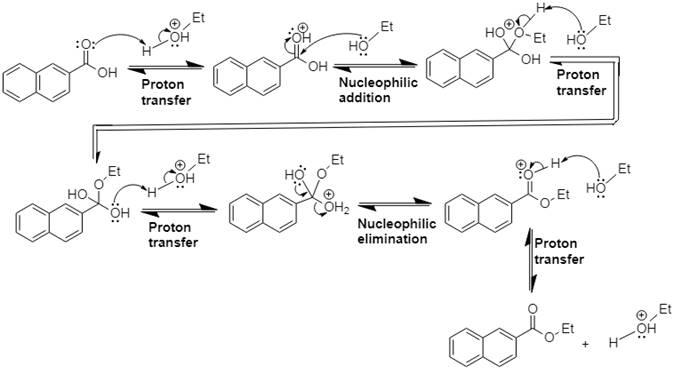
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the acid’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by Fischer esterification reaction.
(f)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of amine. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
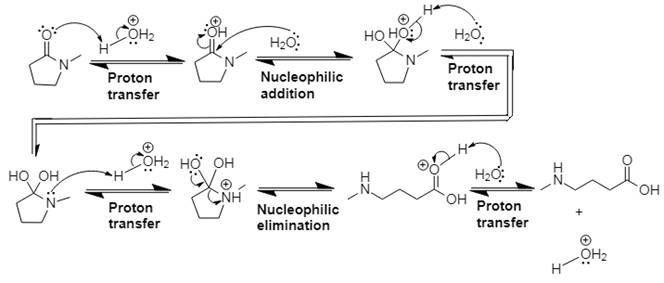
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
Want to see more full solutions like this?
Chapter 21 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
