
Concept explainers
Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interaction energy (the energy required to pull them infinitely far apart) predicted to be larger if the medium between them is water, or if it is n-pentane? (See Table 2.5)
If Ca2+, Na+ and F¯ each have ionic radii ~1.16. Which ionic bond is stronger: Ca-F or Na-F? If Ca2+ is often bound on the surface of a protein by
Table 2.5 Important properties of liquid water compared with those of n-pentane, a nonpolar,
Nonhydrogen-bonding liquida
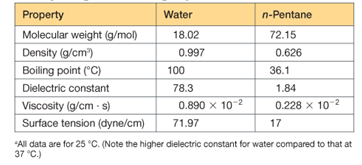
Interpretation:
Interaction energy will be more in which of the given ion pairs must be predicted. The stronger ionic bond must be predicted among the given ionic compounds. In which of the given pH the interaction between
Concept introduction:
Ionic interaction depends on Columbic interaction which depends on the charge of ions and distance by which those ions are separated. It aslo depends on the dielectric constant of the medium and the viscosity of the medium. The ionic bond is stronger when the size of the ions is small, charge in ions is high. Lattice energy depends on Columbic interaction. The bond between
Answer to Problem 1P
The interaction energy between sodium ion and chlorine ion will be larger in case of n-pentane.
Explanation of Solution
The interaction of ion pair will be more in case of n-pentane.
This is because the dielectric constant of n-pentane is
Now,
This is because of Columbic interaction
where
As calcium ion has the double charge of sodium ion so
The
This is because at pH greater than
So, the best pH is
Want to see more full solutions like this?
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
Microbiology with Diseases by Body System (5th Edition)
Anatomy & Physiology (6th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
- Draw the predominant form of glutamic acid at pH = 8.4. The pKa of the side chain is 4.1. Include proper stereochemistry. HO H2N OH pH = 8.4arrow_forwardHow would I draw this?arrow_forwardCalculate the standard change in Gibbs free energy, AGrxn, for the given reaction at 25.0 °C. Consult the table of thermodynamic properties for standard Gibbs free energy of formation values. NH,Cl(s) →NH; (aq) + C1 (aq) AGrxn -7.67 Correct Answer Determine the concentration of NH+ (aq) if the change in Gibbs free energy, AGrxn, for the reaction is -9.27 kJ/mol. 6.49 [NH+] Incorrect Answer kJ/mol Marrow_forward
- What are some topics of interest that neurotoxicologists study? For example, toxin-induced seizures, brain death, and such along those lines?arrow_forwardCould you help me with the explanation of the answer to exercise 15, chapter 1 of Lehinger Question Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo. Identifique los dos carbonos quirales en la siguiente estructura. ¿Es este el(R,R)o el(S,S)¿isómero? Dibuja el otro isómero. Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo.arrow_forwardThe reaction A+B → C + D AG°' = -7.3 kcal/mol can be coupled with which of the following unfavorable reactions to drive it forward? A. EFG+HAG° = 5.6 kcal/mol. B. J+KZ+A AG° = 2.3 kcal/mol. C. P+RY+DAG° = 8.2 kcal/mol. D. C + T → V + W AG°' = -5.9 kcal/mol. E. AN→ Q+KAG°' = 4.3 kcal/mol.arrow_forward
- What would be the toxicological endpoints for neurotoxicity?arrow_forwardWhat are "endpoints" in toxicology exactly? Please give an intuitive easy explanationarrow_forwardFura-2 Fluorescence (Arbitrary Unit) 4500 4000 3500 3000 2500 2000 1500 1000 500 [Ca2+]=2970nM, 25°C [Ca2+] 2970nM, 4°C [Ca2+]=0.9nM, 25°C [Ca2+] = 0.9nM, 4°C 0 260 280 300 340 360 380 400 420 440 Wavelength (nm) ← < The figure on the LHS shows the excitation spectra of Fura-2 (Em = 510 nm) in 2 solutions with two different Ca2+ ion concentration as indicated. Except for temperature, the setting for excitation & signal acquisition was identical.< ப a) The unit in Y-axis is arbitrary (unspecified). Why? < < b) Compare & contrast the excitation wavelength of the Isosbestic Point of Fura-2 at 25 °C & 4 °C. Give a possible reason for the discrepancy. < c) The fluorescence intensity at 25 °C & 4 °C are different. Explain why with the concept of electronic configuration. <arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





