
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 18P
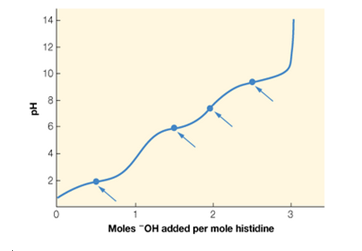
Histidine is an amino acid with three titratable groups: an -NH3+ group (pKa = 9.2) —COOH group (pKa = 1.8). and an imidazole (
a. Identify which point on the titration curve corresponds to the pKa for each of the titratable groups, and which point corresponds to the pl. Explain your choices.
b. Calculate the value of pl for histidine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For a Michaelis-Menten reaction, k₁-5 x 10'/M-s, k.-2 x 10%/s, and k₂-4 x 10²/s.
a)
Calculate the Ks and KM for this reaction.
b)
Does substrate binding achieve equilibrium or steady state?
Assume that an enzyme-catalyzed reaction follows the scheme shown:
E+S SES
→E + P
k₁ = 1 x 109/M-s k-1=2.5 x 10%/s k₂ = 3.4 x 107/s
What is the dissociation constant for the enzyme-substrate, K,?
What is the Michaelis constant, Km, for this enzyme?
What is the turnover number, Keat, for this enzyme?
What is the catalytic efficiency for the enzyme?
If the initial Et concentration is 0.25mM, what is Vmax?
An enzyme lowers the activation energy, (AG) of a reaction from 50.0 kcal/mol to 40.0
kcal/mol. Calulate the catalytic power at 310K. (R-1.987x10 kcal/mol)
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a typical axodendritic synapse, including a specific neurotransmitter of your choice, its associated postsynaptic receptors (indicating whether they are ionotropic or metabotropic), and any associated reuptake transporters or degradation enzymes. Please include a description of what specific steps would occur as an action potential reaches the axonal terminal.arrow_forwardGive a full arrow pushing mechanism of the spontaneous redox reaction between NAD+/NADH and oxaloacetate/malate. Please include diagram drawing of the mechanism! (Thank You!)arrow_forward18. Which one of the compounds below is the major organic product obtained from the following series of reactions? 1. BH3 2. H2O2, NaOH H₂CrO4 CH2N2 oro ororos A B C D Earrow_forward
- 17. Which one of the compounds below is the major organic product obtained from the following series of reactions? CI benzyl alcohol OH PBr3 Mg 1. CO2 SOCl2 ? ether 2. H+, H₂O CI Cl HO OH CI Cl A B C D Earrow_forward14. What is the IUPAC name of this compound? A) 6-hydroxy-4-oxohexanenitrile B) 5-cyano-3-oxo-1-pentanol C) 5-cyano-1-hydroxy-3-pentanone D) 1-cyano-5-hydroxy-3-pentanone E) 5-hydroxy-3-oxopentanenitrile HO. CNarrow_forward13. What is the IUPAC name of this compound? A) 5-hydroxy-3,3-dimethylpentanoic acid B) 3,3-dimethylpentanoic acid C) 3,3-dimethyl-1-oxo-1,5-pentanediol D) 1,5-dihydroxy-3,3-dimethylpentanal E) 4-hydroxy-2,2-dimethylbutanoic acid HO OHarrow_forward
- Help me understand how carbon disulfide leads to toxicity in the brain, using terms like distal axonopathy, neurofilaments, covalent cross-linking, adducts, etc.,...please intuitively explain what is happening and where and the effects of it. For example, I know that CS2 reacts with amide and sulfhydryl groups on proteins, but what proteins exactly and where are they located?arrow_forwardWhat is the standard free energy change (in kJ/mole) of the spontaneous reaction between Oxygen and NADH to form H2O2 and NAD+?arrow_forwardRedox Chemistry: Give standard free energy changes expected for the following reactions:-Succinate -> fumarate (using FAD/FADH2)-Oxaloacetate -> Malate (using NAD/NADH)-NADH --> NAD+ (using FMN/FMNH2)-CoQ --> CoQH2 (using Cytochrome C)arrow_forward
- Give examples of balanced redox reactions that match the following:-Catabolic-Anabolic-Oxidative-Reductivearrow_forwardIf there are 20uM of a GLUT2 transporter on the surface of a cell, each able to move 8 per second, and 50mM glucose outside of the cell, what is the flux into the cell in mM/sec?arrow_forwardA transporter is responsible for antiporting calcium and glucose. The transporter brings glucose into the cell and sends calcium out of the cell. If blood [calcium] = 2.55mM and intracellular [calcium] = 7uM, blood [glucose] = 5.2mM, and intracellular [glucose] = 40uM, what is the free energy of transport? Assume a membrane potential of 62mV (negative inside).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY