
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 15P
The anno acid arginine ionizes according to the following scheme:
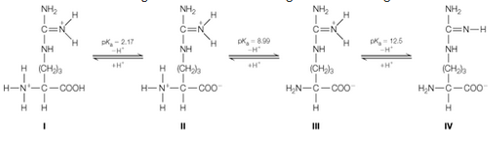
a. Calculate the isoelectric point of arginine. You can neglect contributions from form l. Why?
b. Calculate the average charge on arginine when pH = 9.20. (Hint: Find the average charge for each ionizable group and sum these together)
c. Is the value of average charge you calculated in part b reasonable, given the pl you calculated part a? Explain your answer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the chemical structure of the
tripeptide, HEL (L - amino acids), at
pH =
7.0. Calculate isoelectric point
Can someone draw what this would look like?
How would I fix this?
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the predominant form of glutamic acid at pH = 8.4. The pKa of the side chain is 4.1. Include proper stereochemistry. HO H2N OH pH = 8.4arrow_forwardHow would I draw this?arrow_forwardCalculate the standard change in Gibbs free energy, AGrxn, for the given reaction at 25.0 °C. Consult the table of thermodynamic properties for standard Gibbs free energy of formation values. NH,Cl(s) →NH; (aq) + C1 (aq) AGrxn -7.67 Correct Answer Determine the concentration of NH+ (aq) if the change in Gibbs free energy, AGrxn, for the reaction is -9.27 kJ/mol. 6.49 [NH+] Incorrect Answer kJ/mol Marrow_forward
- What are some topics of interest that neurotoxicologists study? For example, toxin-induced seizures, brain death, and such along those lines?arrow_forwardCould you help me with the explanation of the answer to exercise 15, chapter 1 of Lehinger Question Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo. Identifique los dos carbonos quirales en la siguiente estructura. ¿Es este el(R,R)o el(S,S)¿isómero? Dibuja el otro isómero. Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo.arrow_forwardThe reaction A+B → C + D AG°' = -7.3 kcal/mol can be coupled with which of the following unfavorable reactions to drive it forward? A. EFG+HAG° = 5.6 kcal/mol. B. J+KZ+A AG° = 2.3 kcal/mol. C. P+RY+DAG° = 8.2 kcal/mol. D. C + T → V + W AG°' = -5.9 kcal/mol. E. AN→ Q+KAG°' = 4.3 kcal/mol.arrow_forward
- What would be the toxicological endpoints for neurotoxicity?arrow_forwardWhat are "endpoints" in toxicology exactly? Please give an intuitive easy explanationarrow_forwardFura-2 Fluorescence (Arbitrary Unit) 4500 4000 3500 3000 2500 2000 1500 1000 500 [Ca2+]=2970nM, 25°C [Ca2+] 2970nM, 4°C [Ca2+]=0.9nM, 25°C [Ca2+] = 0.9nM, 4°C 0 260 280 300 340 360 380 400 420 440 Wavelength (nm) ← < The figure on the LHS shows the excitation spectra of Fura-2 (Em = 510 nm) in 2 solutions with two different Ca2+ ion concentration as indicated. Except for temperature, the setting for excitation & signal acquisition was identical.< ப a) The unit in Y-axis is arbitrary (unspecified). Why? < < b) Compare & contrast the excitation wavelength of the Isosbestic Point of Fura-2 at 25 °C & 4 °C. Give a possible reason for the discrepancy. < c) The fluorescence intensity at 25 °C & 4 °C are different. Explain why with the concept of electronic configuration. <arrow_forward
- draw in the structure of each amino acid (as L-amino acids) using the Fischer projection style. an example has been included. Draw the structure for glycine, alanine, valine, isoleucine, methionine, proline, phenylalanine, tryptophan, serine, threonine, asparagine, glutamine, lysine, arginine, aspartic acid, glutamic acid, histidine, tyrosine, cysteinearrow_forwarddraw in the structure of each amino acid (as L-amino acids) using the Fischer projection style. an example has been includedarrow_forwarddraw in the structure of each amino acid (as L-amino acids) using the Fischer projection style. an example has been includedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning



Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license