
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 11P
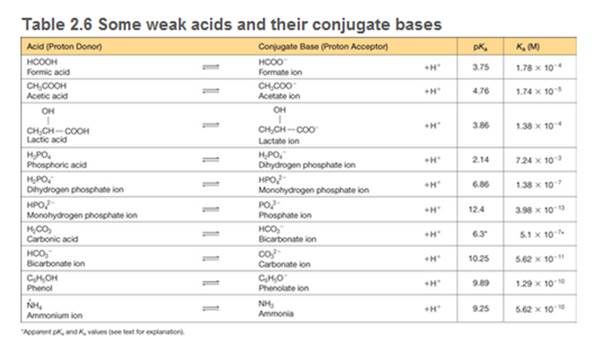
You need to make a buffer whose pH is 7.0, and you can choose from the weak acids shown in Table 2.6. Briefly explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Here is my literature Beta Carotene HPLC analysis graph.
Can you help me explain what each peak is at each retention time?
Thank You :D
I have a literature B-Carotene HPLC graph in which showcases a retention time of roughly 23.6 and 25.1.
Please help me compare my two different Anti-Oxidant Juice graphs. (Attached)
The juices provided are: V8 Carrot Ginger Blend and V8 Original Blend
Noticing the HPLC graphs I saw no peaks for the Original Blend for B-Carotene.
However the Carrot Ginger Blend showed similar peaks --> Why is this reason?
Please explain in terms of Retention time and Area (Under Curve).
Thank You!
Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Show a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forwardDon't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forward
- Problem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward1:30 5G 47% Problem 10 of 15 Submit Using the following reaction data points, construct a Lineweaver-Burk plot for an enzyme with and without a competitive inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. 1 -1 1 mM [S]' s mM¹ with 10 mg pe 20 V' 54 10 36 > ст 5 27 2.5 23 1.25 20 Answer: |||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning



Essentials of Pharmacology for Health Professions
Nursing
ISBN:9781305441620
Author:WOODROW
Publisher:Cengage

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning


GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license