
Concept explainers
a)
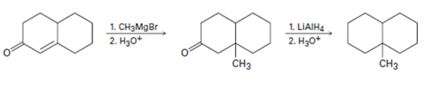
Interpretation:
The flaw in the reaction scheme given is to be identified and the corrected scheme is to be provided.
Concept introduction:
α,β-Unsaturated
To identify:
The flaw in the reaction scheme given and to provide the corrected scheme.
b)

Interpretation:
The flaw in the reaction scheme given is to be identified and corrected scheme is to be given.
Concept introduction:
CrO3 in the presence of H3O+ oxidize primary alcohols to acids and not to
To identify:
The flaw in the reaction scheme given and to provide the corrected scheme.
c)
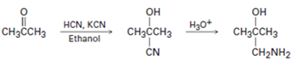
Interpretation:
The flaw in the reaction scheme given is to be identified and corrected scheme is to be given.
Concept introduction:
Aldehydes and ketones react with HCN, KCN to yield the corresponding cyanohydrins. The cyanohydrins when treated with aqueous acids will get hydrolysed to acids.
To identify:
The flaw in the reaction scheme given and to provide the corrected scheme.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

