
a)
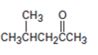
Interpretation:
How to prepare 4-methylpentan-2-one from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The double bond in an unsaturated
To show:
How to prepare 4-methylpentan-2-one from 4-methyl-3-pentene-2-one.
b)
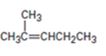
Interpretation:
How to prepare 2-methylpent-2-ene from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The carbonyl group in a unsaturated ketone can be reduced to –CH2 without affecting the double bond by treating it with hydrazine in the presence of KOH (Wolf-Kishner reduction).
To show:
How to prepare 2-methylpent-2-ene from 4-methyl-3-pentene-2-one.
c)
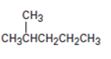
Interpretation:
How to prepare 2-methylpentane from 4-methyl-3-pentene-2-one is to be shown.
Concept introduction:
The double bond in an unsaturated ketone is reduced without affecting the carbonyl group by treating it with H2/Pd. The carbonyl group of the saturated ketone formed can be converted to –CH2 group by Wolff-Kishner reduction.
To show:
How to prepare 2-methylpentane from 4-methyl-3-pentene-2-one.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- Can I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forwardCan I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forward
- Draw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forwardWhat is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forward
- Identify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward4:45 PM Tue Apr 1 K 77% Problem 9 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. :0: H Select to Add Arrows HI CH3OH H+ ·HO CH3OH, H+ 0:0 H H Select to Add Arrows tion Versirate CH3OH, H* Select to Draw Productarrow_forwardCan I please get help with this graph? If you can show exactly where it needs to pass through.arrow_forward
- G 1. PPh3, THF 2. 3. LiH, THF ' THF H Harrow_forwardPlease EnCircle or Fill-In your Choice CLEARLY: 21. Please Sketch the intermediates for each step below. Draw the Product which would result from the following series of reactions. Name each Type of Rx: 1. Br2, FeBr3 2. Mg, ether 3. ethylene oxide 4. H₂O+ 5. PBr3 6. Mg, ether 7. 8. H3O+, heat (-H₂O 9. HF ?arrow_forwardCan I please get help with this question. All required information should be in data table.arrow_forward
