
Concept explainers
a)
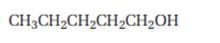
Interpretation:
Starting from 1-pentanol how pentanal can be prepared is to be shown.
Concept introduction:
Primary alcohols are oxidized to carboxylic acids by strong oxidizing agents like CrO3 and KMnO4 etc. The oxidation can be stopped in the
To show:
How to prepare pentanal starting from 1-pentanol.
b)
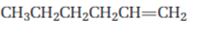
Interpretation:
Starting from 1-hexene how pentanal can be prepared is to be shown.
Concept introduction:
When treated with ozone,
To show:
How to prepare pentanal starting from 1-hexene.
c)
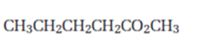
Interpretation:
Starting from methylpentanoate how pentanal can be prepared is to be shown.
Concept introduction:
To show:
How to prepare pentanal starting from methylpentanoate.
d)
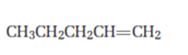
Interpretation:
Starting from 1-pentene how pentanal can be prepared is to be shown.
Concept introduction:
Alkenes when subjected to hydroboration-oxidation yield primary alcohols which when oxidized with Dess-Martin periodinate in dichloromethane yield aldehydes.
To show:
How to prepare pentanal starting from 1-pentene.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- b) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forwardQ2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forwardd) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forward
- Explain why the S-F bond strength is 367 kJ/mol in SF2 and 329 kJ/mol in SF6.arrow_forwardWould Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.arrow_forwardNH3 reacts with boron halides (BX3 where X = F, Cl, Br, or I) to form H3N-BX3 complexes.Which of these complexes will have the strongest N-B bond? Justify your answerarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





