
Concept explainers
(a)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an
aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain
alkane is replaced by “-amine”. - Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(a)
Answer to Problem 17.57EP
Name of the parent amine is 1-propanamine.
Explanation of Solution
Given amine salt is
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,

Structure of the amine is,
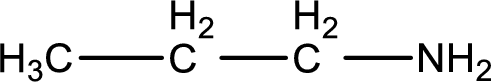
The longest carbon chain attached to the nitrogen atom is found to be containing three carbon atoms. Hence, the parent alkane is propane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as propanamine.
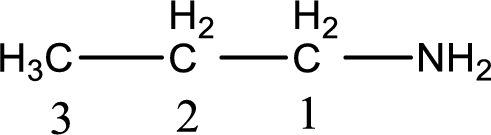
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 1. This has to be added to the name in front. This gives the IUPAC name of 1-propanamine.
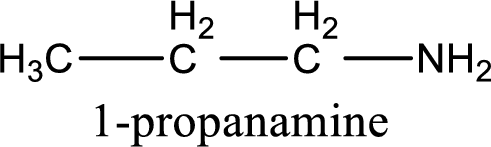
IUPAC name for the parent amine is given.
(b)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(b)
Answer to Problem 17.57EP
Name of the parent amine is N-methyl-1-propanamine.
Explanation of Solution
Given amine salt is,
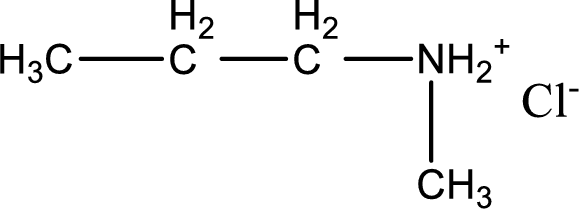
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
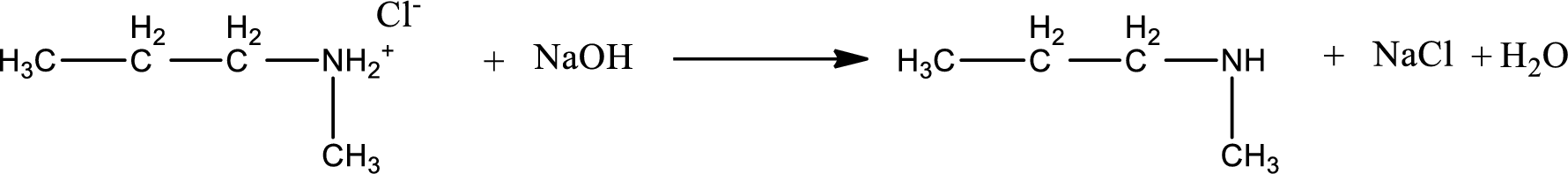
Structure of the amine is,
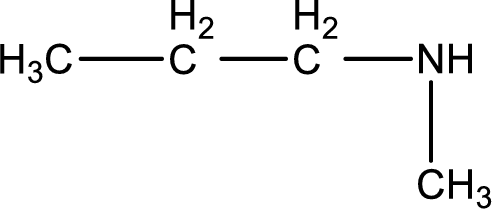
The longest carbon chain attached to the nitrogen atom is found to be containing three carbon atoms. Hence, the parent alkane is propane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as propanamine.
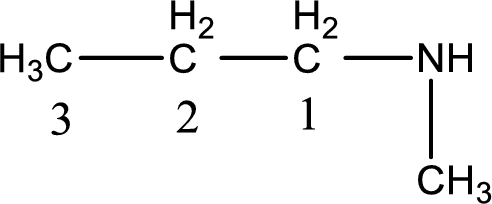
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 1. This has to be added to the name in front. Looking for substituent a methyl group is present on the nitrogen atom. This gives the IUPAC name of N-methyl-1-propanamine.
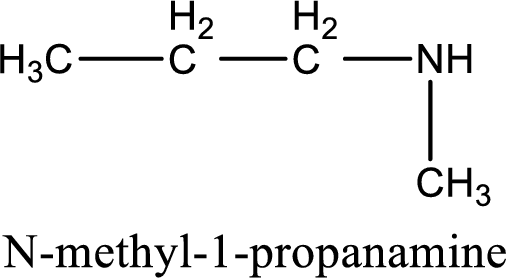
IUPAC name for the parent amine is given.
(c)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(c)
Answer to Problem 17.57EP
Name of the parent amine is N,N-dimethylethanamine.
Explanation of Solution
Given amine salt is,
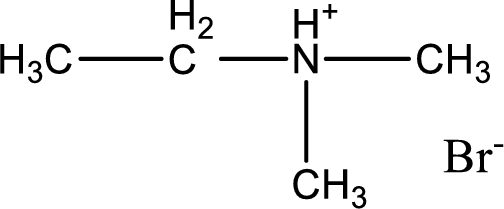
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
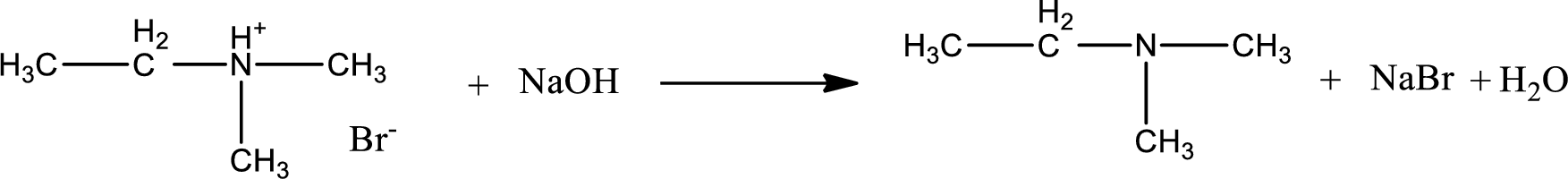
Structure of the amine is,
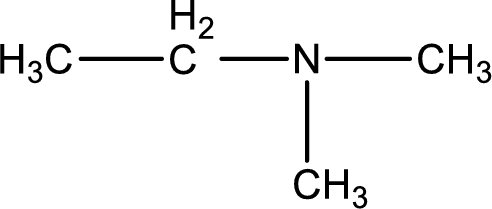
The longest carbon chain attached to the nitrogen atom is found to be containing two carbon atoms. Hence, the parent alkane is ethane. Amine is named by replacing the suffix “-e” in the parent alkane name with “-amine”. This gives the name as ethanamine.
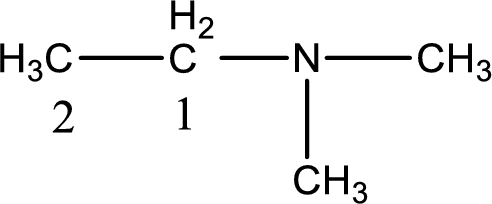
Numbering has to be given from the end that is near to the nitrogen atom. In this case, the nitrogen atom is attached to the carbon atom that is numbered 1. In this case it is not necessary to add the number as only two carbon atoms are present. Looking for substituents, two methyl groups are present on the nitrogen atom. This gives the IUPAC name of N,N-dimethylethanamine.
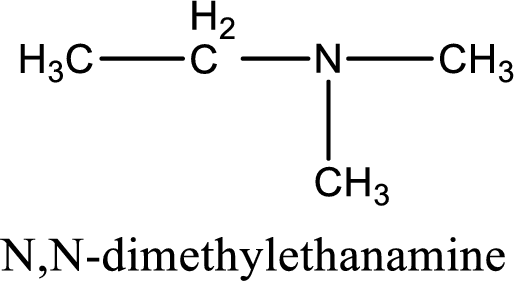
IUPAC name for the parent amine is given.
(d)
Interpretation:
IUPAC name for the parent amine from which the given amine salt is formed has to be given.
Concept Introduction:
IUPAC rules for naming ammonium ions:
There are two rules that has to be followed while naming a positive ion (ammonium ion) and they are,
- In order to name an alkylamine, the ending of the name amine is changed from amine to ammonium ion.
- In order to name an aromatic amine, the ending of the name “-e” is replaced by “-ium ion”.
IUPAC nomenclature for amine: There are about five rules to be followed in giving IUPAC name for an amine.
- Longest carbon chain has to be identified that is attached to nitrogen atom.
- Suffix “-e” in name of the parent chain alkane is replaced by “-amine”.
- Numbering of the carbon chain is done from the end that is near the nitrogen atom.
- Point of attachment of the nitrogen atom in the carbon chain is indicated by a number before the parent chain name.
- In case if substituents are present, then the identity and location of substituents are appended to the front in the parent chain name.
If the compound contains two amine groups, then the suffix “-e” is replaced by diamine. Tertiary and secondary amines are named as N-substituted primary amines.
Common name for amine is given in a single word. Primary amine is named as alkylamine. Secondary amine is named as alkylalkylamine. Tertiary amine is named as alkylalkylalkylamine.
(d)
Answer to Problem 17.57EP
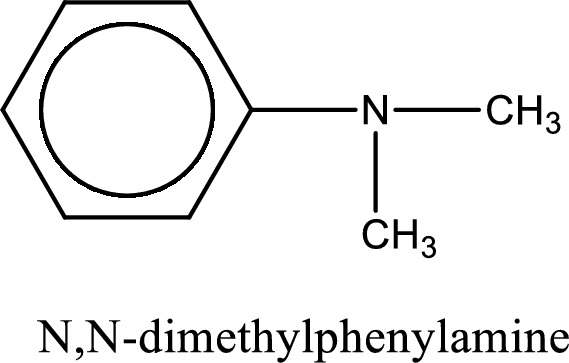
Name of the parent amine is N,N-dimethylphenylamine.
Explanation of Solution
Given amine salt is,
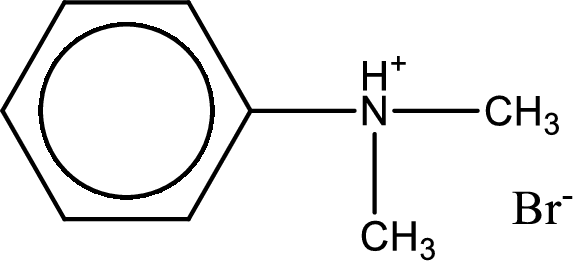
The parent amine can be found by deprotonating the amine salt. This can be accomplished by treating it with a strong base. The complete reaction can be given as,
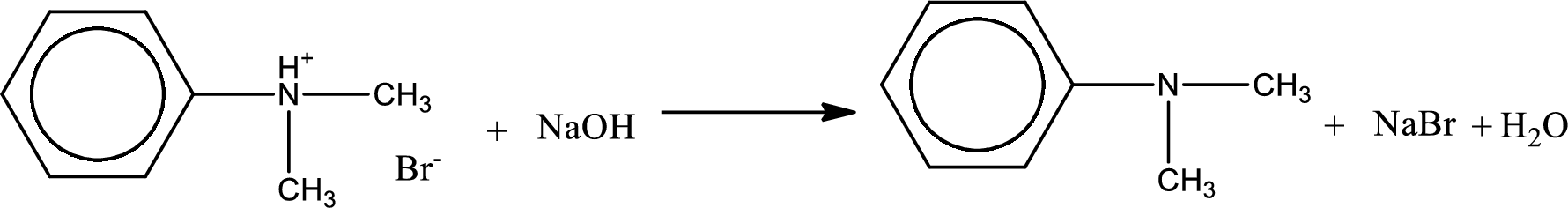
Structure of the amine is,
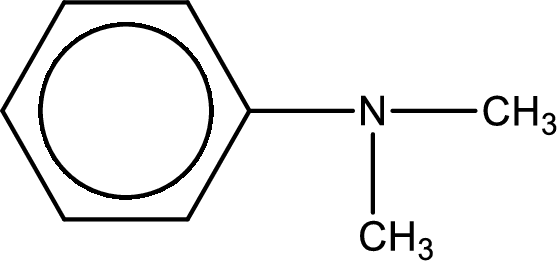
The longest carbon chain attached to the nitrogen atom is found to be containing six carbon cyclic chain. Hence, the parent is benzene ring. This has to be named as phenylamine.
Looking for substituents, two methyl groups are present on the nitrogen atom. This gives the IUPAC name of N,N-dimethylphenylamine.
IUPAC name for the parent amine is given.
Want to see more full solutions like this?
Chapter 17 Solutions
General, Organic, and Biological Chemistry
- If a bacterium using aerobic respiration was to degrade one small protein molecule into 8 molecules of pyruvic acid, how many ATP would that cell make? Assume there is no other carbon source. Units cannot be entered in this style of question but the units of your answer should be in molecules of ATP.arrow_forwardIf a bacterium using aerobic respiration was to degrade a 30 mM solution of citric acid, how many ATP would that cell make? Assume no other carbon source is available. Units cannot be entered in this style of question but the units of your answer should be in mM of ATP.arrow_forwardHow much ATP will be produced during the following metabolic scenario: Aerobic respiration of a 5mM lipid solution that is made up of one glycerol and an 8-carbon fatty acid and 12-carbon fatty acid. Recall that when glycerol breaks down to Glyceraldehyde-3-phosphate it costs one ATP but your get an extra FADH2. Every two carbons of a fatty acid break down to one acetyl-CoA. (pathways will be provided on the exam) Units cannot be entered in this style of question but the units of your answer should be in mM of ATP.arrow_forward
- When beta-lactamase was isolated from Staphylcoccus aureus and treated with a phosphorylating agent, only the active site, serine was phosphorylated. Additionally, the serine was found to constitute 0.35% (by weight) of this beta-lactamase enzyme. Using this, calculate the molecular weight of this enzyme and estimate the number of amino acids present in the polypeptide.arrow_forwardBased on your results from the Mannitol Salt Agar (MSA) media, which of your bacteria were mannitol fermenters and which were not mannitol fermenters?arrow_forwardhelp tutor pleasearrow_forward
- Q8. A researcher wants to study the effectiveness of a pill intended to reduce stomach heartburn in pregnant women. The researcher chooses randomly 400 women to participate in this experiment for 9 months of their pregnancy period. They all need to have the same diet. The researcher designs two groups of 200 participants: One group take the real medication intended to reduce heartburn, while the other group take placebo medication. In this study what are: Independent variable: Dependent variable: Control variable: Experimental group: " Control group: If the participants do not know who is consuming the real pills and who is consuming the sugar pills. This study is It happens that 40% of the participants do not find the treatment helpful and drop out after 6 months. The researcher throws out the data from subjects that drop out. What type of bias is there in this study? If the company who makes the medication funds this research, what type of bias might exist in this research work?arrow_forwardHow do I determine the inhertiance pattern from the pedigree diagram?arrow_forwardits an open book assignemntarrow_forward
- Describe two different gene regulation mechanisms involving methylationarrow_forwardWhat is behavioral adaptarrow_forward22. Which of the following mutant proteins is expected to have a dominant negative effect when over- expressed in normal cells? a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function b. mutant Grb2 protein that cannot bind to RTK c. mutant RTK that lacks the extracellular domain d. mutant PDK that has the PH domain but lost the kinase function e. all of the abovearrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage





