
Concept explainers
(a)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
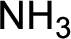
Explanation of Solution
Given structure of compound is,
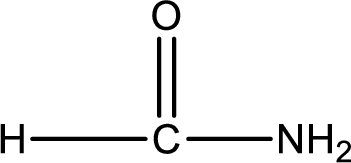
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
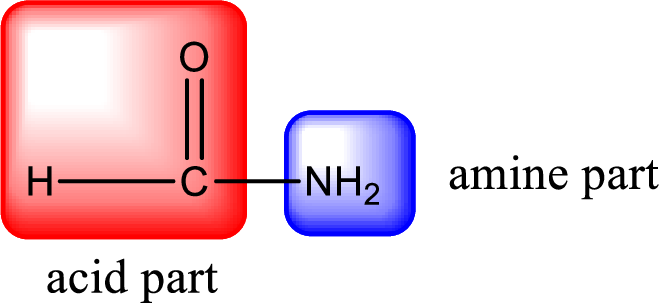
Hydrogen atom has to be added to the amine part and
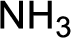
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(b)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
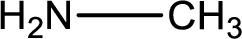
Explanation of Solution
Given structure of compound is,
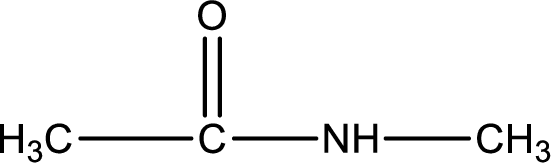
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
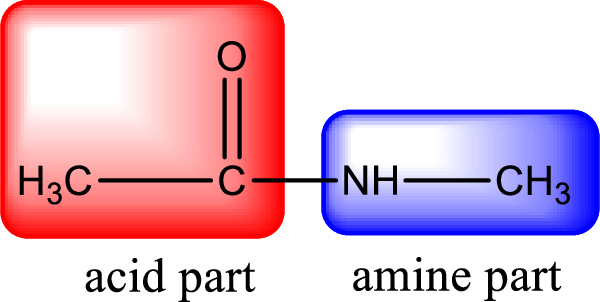
Hydrogen atom has to be added to the amine part and
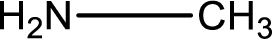
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(c)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
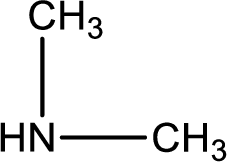
Explanation of Solution
Given structure of compound is,
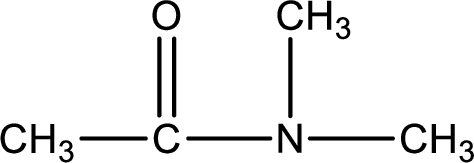
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
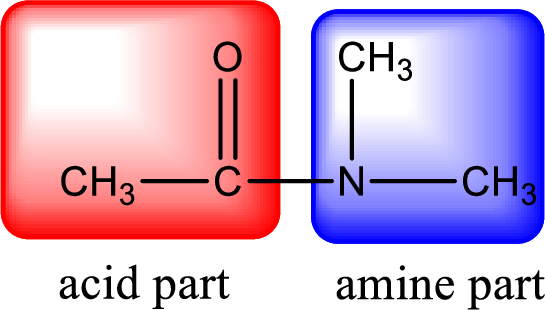
Hydrogen atom has to be added to the amine part and
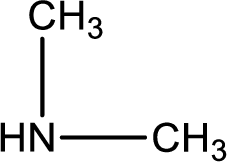
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(d)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 17.136EP
Nitrogen-containing compound that is required was,
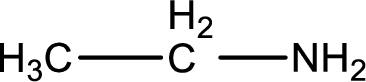
Explanation of Solution
Given structure of compound is,
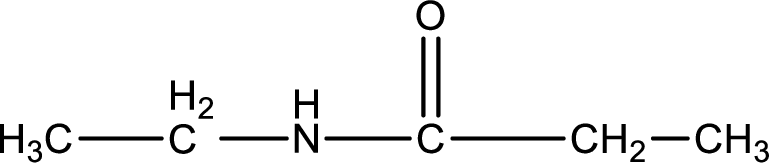
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
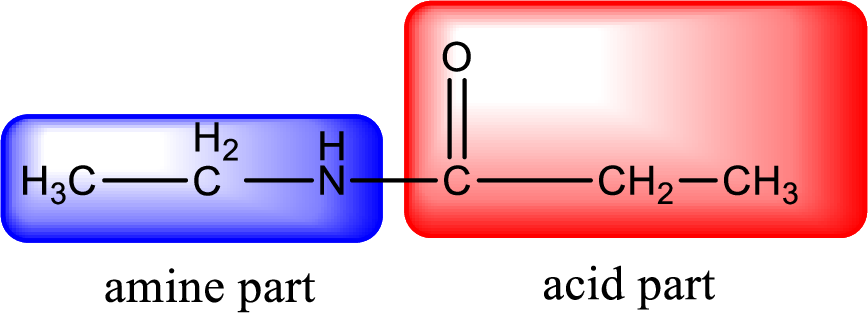
Hydrogen atom has to be added to the amine part and
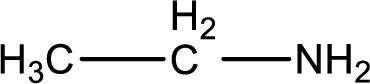
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
General, Organic, and Biological Chemistry
- In one paragraph show how atoms and they're structure are related to the structure of dna and proteins. Talk about what atoms are. what they're made of, why chemical bonding is important to DNA?arrow_forwardWhat are the structure and properties of atoms and chemical bonds (especially how they relate to DNA and proteins).arrow_forwardThe Sentinel Cell: Nature’s Answer to Cancer?arrow_forward
- Molecular Biology Question You are working to characterize a novel protein in mice. Analysis shows that high levels of the primary transcript that codes for this protein are found in tissue from the brain, muscle, liver, and pancreas. However, an antibody that recognizes the C-terminal portion of the protein indicates that the protein is present in brain, muscle, and liver, but not in the pancreas. What is the most likely explanation for this result?arrow_forwardMolecular Biology Explain/discuss how “slow stop” and “quick/fast stop” mutants wereused to identify different protein involved in DNA replication in E. coli.arrow_forwardMolecular Biology Question A gene that codes for a protein was removed from a eukaryotic cell and inserted into a prokaryotic cell. Although the gene was successfully transcribed and translated, it produced a different protein than it produced in the eukaryotic cell. What is the most likely explanation?arrow_forward
- Molecular Biology LIST three characteristics of origins of replicationarrow_forwardMolecular Biology Question Please help. Thank you For E coli DNA polymerase III, give the structure and function of the b-clamp sub-complex. Describe how the structure of this sub-complex is important for it’s function.arrow_forwardMolecular Biology LIST three characteristics of DNA Polymerasesarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





