
Concept explainers
(a)
Interpretation:
IUPAC name for the
Concept Introduction:
For naming a carboxylic acid in
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for
aldehyde , the carboxyl carbon is always numbered 1. - The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl
functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
(a)
Answer to Problem 16.16EP
IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
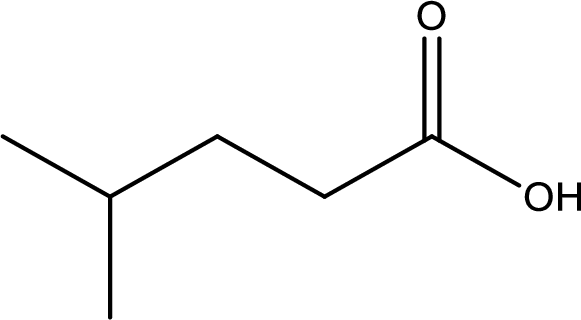
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a five carbon chain. Hence, the parent alkane is pentane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as pentanoic acid.
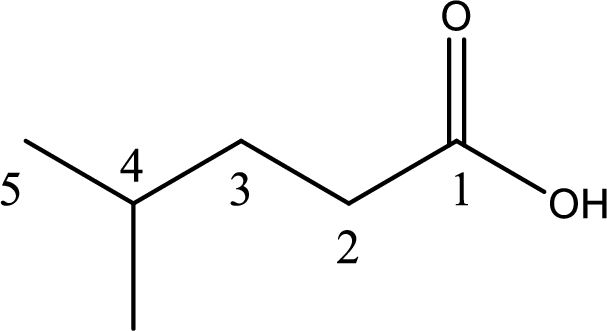
Looking for substituents it is found that there is a methyl group present on fourth carbon atom. Hence, the IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
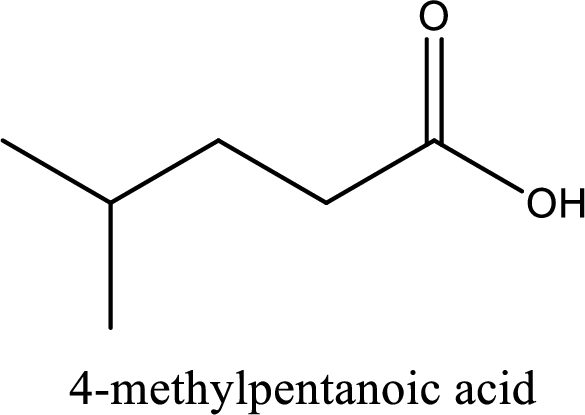
The IUPAC name of the given carboxylic acid is 4-methylpentanoic acid.
IUPAC name of the given carboxylic acid is found out.
(b)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for carboxylic acid, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
(b)
Answer to Problem 16.16EP
IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
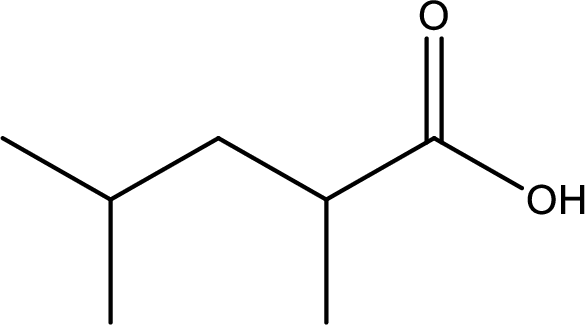
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a five carbon chain. Hence, the parent alkane is pentane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as pentanoic acid.
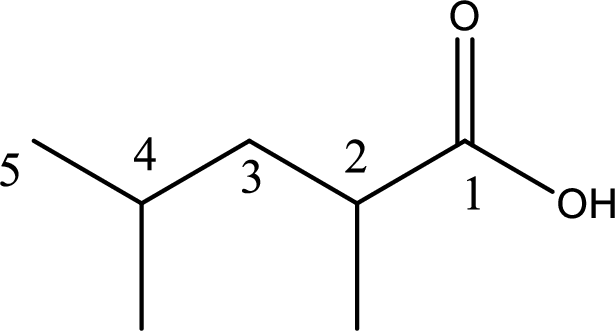
Looking for substituents it is found that there are two methyl groups present, each on second and fourth carbon atom. In the IUPAC name prefix “-di” has to be added before the alkyl name. Hence, the IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
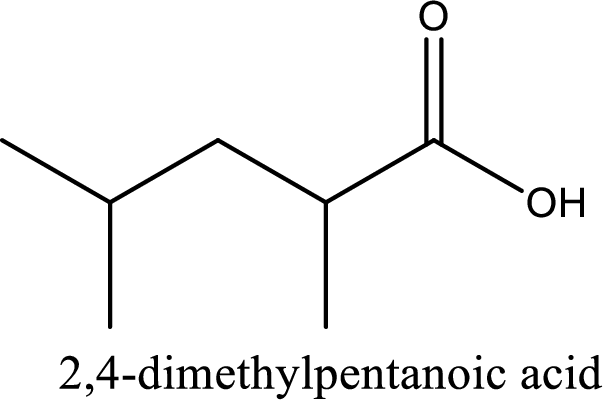
The IUPAC name of the given carboxylic acid is 2,4-dimethylpentanoic acid.
IUPAC name of the given carboxylic acid is found out.
(c)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
(c)
Answer to Problem 16.16EP
IUPAC name of the given carboxylic acid is heptanoic acid.
Explanation of Solution
Given structure of carboxylic acid is,
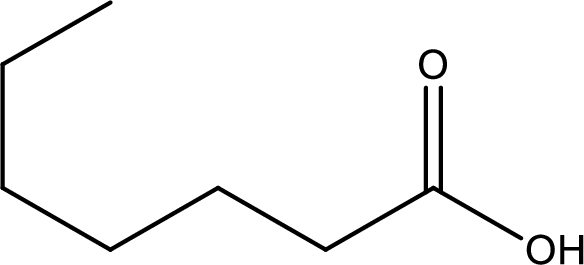
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a seven carbon chain. Hence, the parent alkane is heptane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as heptanoic acid.
Looking for substituents it is found that there are no substituents present on the carbon chain. Hence, the IUPAC name of the given carboxylic acid is heptanoic acid.
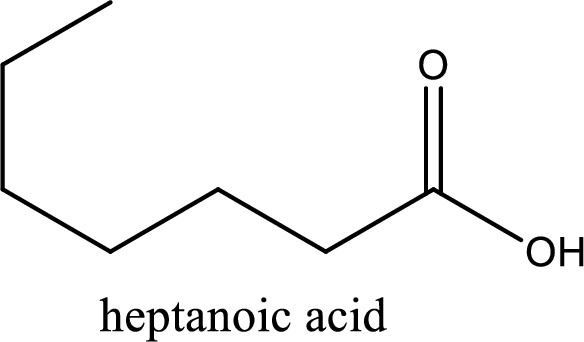
The IUPAC name of the given carboxylic acid is heptanoic acid.
IUPAC name of the given carboxylic acid is found out.
(d)
Interpretation:
IUPAC name for the carboxylic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
In a line-angle structural formula the point at which two lines intersect and the end points are carbon atoms.
(d)
Answer to Problem 16.16EP
IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
Explanation of Solution
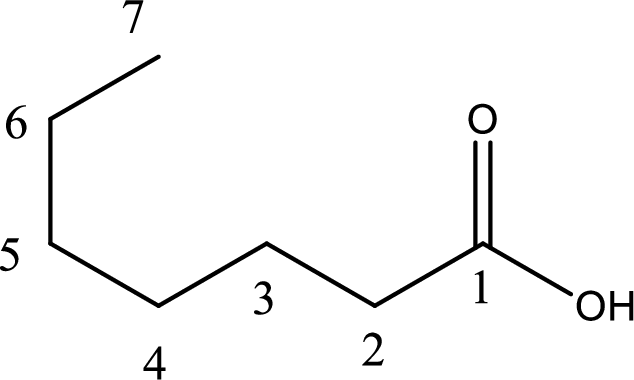
Given structure of carboxylic acid is,
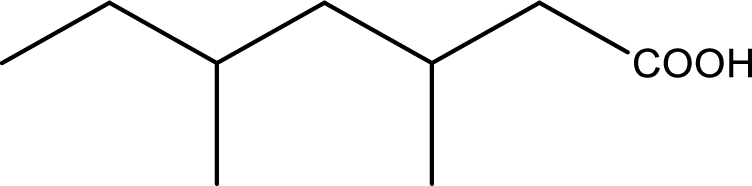
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a seven carbon chain. Hence, the parent alkane is heptane. The carboxylic acid is named by replacing the suffix “-e” in the alkane name with “-oic acid”. This gives the name of carboxylic acid as heptanoic acid.
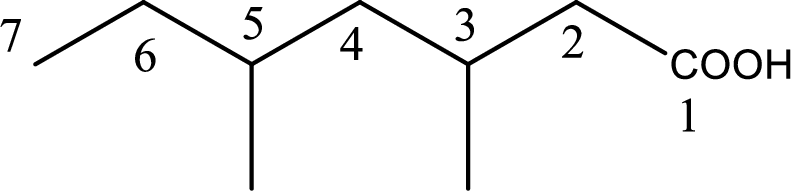
Looking for substituents it is found that there are two methyl groups present as substituents, each on third carbon atom and fifth carbon atom. Hence, the IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
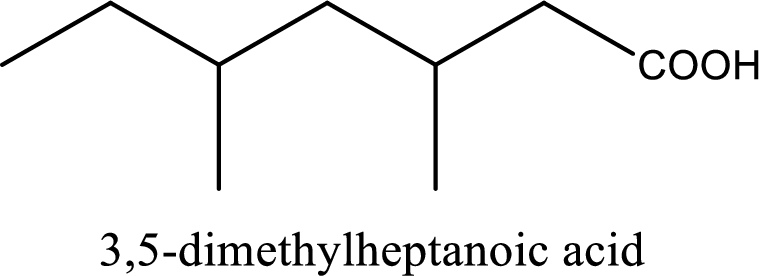
The IUPAC name of the given carboxylic acid is 3,5-dimethylheptanoic acid.
IUPAC name of the given carboxylic acid is found out.
Want to see more full solutions like this?
Chapter 16 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
- Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

