
Concept explainers
(a)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
For naming an acid anhydride, it can be structurally viewed in a way that contains two carbonyl groups that is joined by a single oxygen atom. This can also be said as two acyl group joined by a single oxygen atom.
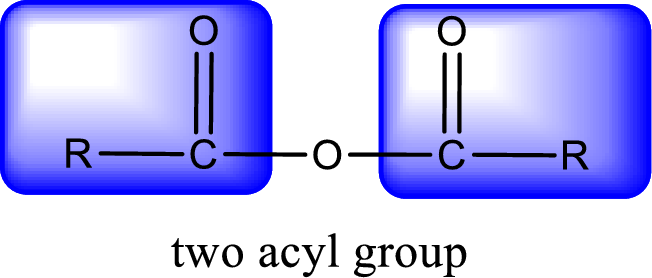
Rules to obtain IUPAC name and common name for an acid anhydride:
- IUPAC name and common name for Symmetric anhydride is obtained by replacing the acid present in the name of parent
carboxylic acid with the word anhydride. - IUPAC name and common name for mixed anhydride is obtained by using the names of the parent carboxylic acids arranged in alphabetical order that is followed by the word anhydride.
(b)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
For naming an acid chloride, it can be structurally viewed in a way that contains one acyl group with a chlorine atom bonded to the carbonyl group
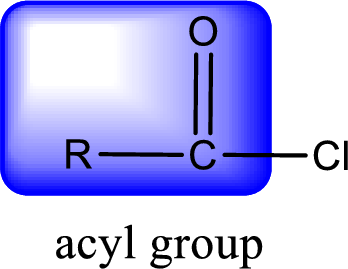
Rules to obtain IUPAC name and common name for an acid chloride:
- IUPAC name for acid chloride can be obtained from the parent carboxylic acid name. In the parent carboxylic acid name, the ending “-oic acid” is replaced by “-oyl chloride”.
- Common name for acid chloride can be obtained from the parent carboxylic acid name. In the parent carboxylic acid name, the ending “-ic acid” is replaced by “-yl chloride”.
(c)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
For naming an acid chloride, it can be structurally viewed in a way that contains one acyl group with a chlorine atom bonded to the carbonyl group
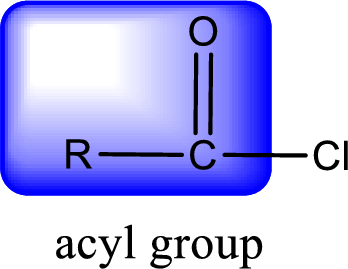
Rules to obtain IUPAC name and common name for an acid chloride:
- IUPAC name for acid chloride can be obtained from the parent carboxylic acid name. In the parent carboxylic acid name, the ending “-oic acid” is replaced by “-oyl chloride”.
- Common name for acid chloride can be obtained from the parent carboxylic acid name. In the parent carboxylic acid name, the ending “-ic acid” is replaced by “-yl chloride”.
(d)
Interpretation:
IUPAC name for the given compound has to be assigned.
Concept Introduction:
For naming an acid anhydride, it can be structurally viewed in a way that contains two carbonyl groups that is joined by a single oxygen atom. This can also be said as two acyl group joined by a single oxygen atom.
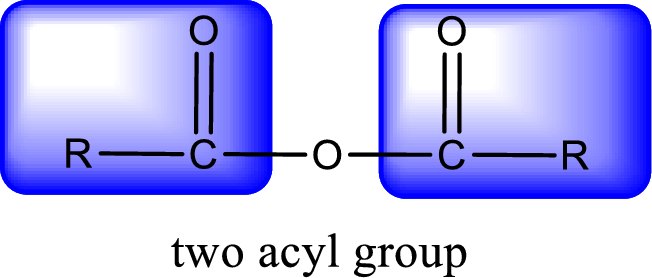
Rules to obtain IUPAC name and common name for an acid anhydride:
- IUPAC name and common name for Symmetric anhydride is obtained by replacing the acid present in the name of parent carboxylic acid with the word anhydride.
- IUPAC name and common name for mixed anhydride is obtained by using the names of the parent carboxylic acids arranged in alphabetical order that is followed by the word anhydride.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


