
(a)
Interpretation:
The noncarboxyl
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
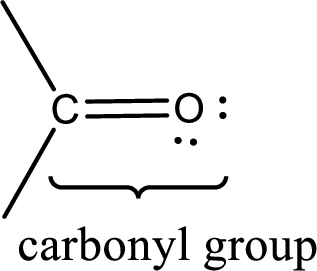
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
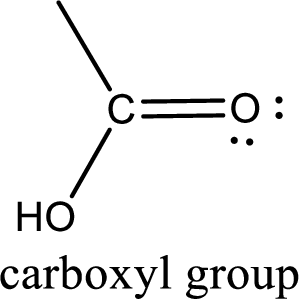
Apart from carboxyl group, there can be other functional group also that is present in the
(b)
Interpretation:
The noncarboxyl functional group that is present in lactic acid has to be given.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
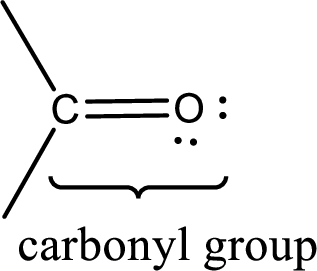
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
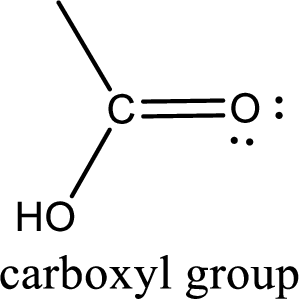
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
(c)
Interpretation:
The noncarboxyl functional group that is present in maleic acid has to be given.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
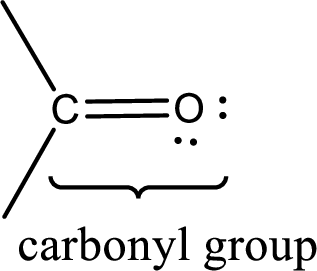
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
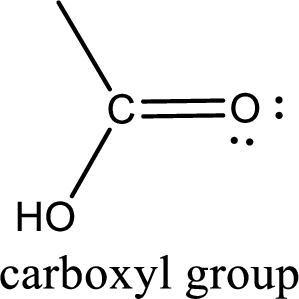
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
(d)
Interpretation:
The noncarboxyl functional group that is present in glycolic acid has to be given.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
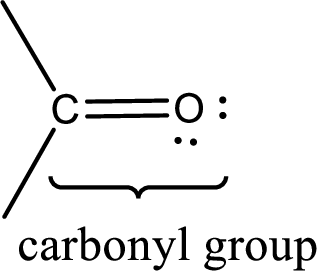
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
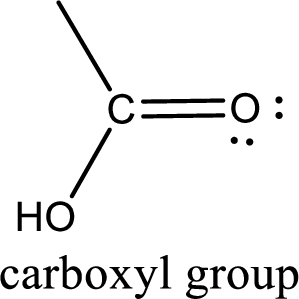
Apart from carboxyl group, there can be other functional group also that is present in the carboxylic acid. They are known as polyfunctional carboxylic acids. These occur naturally also. Some of the important type of polyfunctional carboxylic acids are hydroxy acids, keto acids, and unsaturated acids.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning


