
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
7th Edition
ISBN: 9781305866966
Author: STOKER
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15.8, Problem 2QQ
Interpretation Introduction
Interpretation:
The hydrogen bonding is possible in which of the given case has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
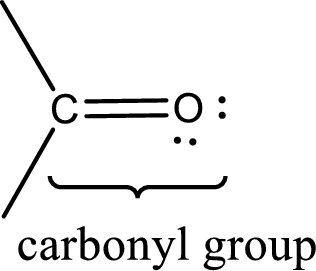
Aldehydes and ketones do not have hydrogen bonding within other molecules of same kind. They have only dipole-dipole interaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Recognizing ampli
Draw an a amino acid with a methyl (-CH3) side chain.
Explanation
Check
Click and drag to start drawing a
structure.
X
C
Write the systematic name of each organic molecule:
structure
name
×
HO
OH
☐
OH
CI
CI
O
CI
OH
OH
く
Check the box under each a amino acid.
If there are no a amino acids at all, check the "none of them" box under the table.
Note for advanced students: don't assume every amino acid shown must be found in nature.
COO
H3N-C-H
CH2
HO
CH3
NH3 O
CH3-CH
CH2
OH
Onone of them
Explanation
Check
+
H3N
O
0.
O
OH
+
NH3
CH2
CH3-CH
H2N C-COOH
H
O
HIC
+
C=O
H3N-C-O
CH3- - CH
CH2
OH
Х
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acces
Chapter 15 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
Ch. 15.1 - Prob. 1QQCh. 15.1 - Prob. 2QQCh. 15.2 - Prob. 1QQCh. 15.2 - Prob. 2QQCh. 15.2 - Prob. 3QQCh. 15.3 - Prob. 1QQCh. 15.3 - Prob. 2QQCh. 15.3 - Prob. 3QQCh. 15.4 - Prob. 1QQCh. 15.4 - Prob. 2QQ
Ch. 15.4 - Prob. 3QQCh. 15.4 - Prob. 4QQCh. 15.4 - Prob. 5QQCh. 15.5 - Prob. 1QQCh. 15.5 - Prob. 2QQCh. 15.5 - Prob. 3QQCh. 15.5 - Prob. 4QQCh. 15.5 - Prob. 5QQCh. 15.6 - Prob. 1QQCh. 15.6 - Prob. 2QQCh. 15.6 - Prob. 3QQCh. 15.7 - Prob. 1QQCh. 15.7 - Prob. 2QQCh. 15.8 - Prob. 1QQCh. 15.8 - Prob. 2QQCh. 15.9 - Prob. 1QQCh. 15.9 - Prob. 2QQCh. 15.10 - Prob. 1QQCh. 15.10 - Prob. 2QQCh. 15.10 - Prob. 3QQCh. 15.10 - Prob. 4QQCh. 15.11 - Prob. 1QQCh. 15.11 - Prob. 2QQCh. 15.11 - Prob. 3QQCh. 15.11 - Prob. 4QQCh. 15.11 - Prob. 5QQCh. 15.12 - Prob. 1QQCh. 15.12 - Prob. 2QQCh. 15 - Prob. 15.1EPCh. 15 - Prob. 15.2EPCh. 15 - Prob. 15.3EPCh. 15 - In terms of polarity, which carbonyl group atom...Ch. 15 - Prob. 15.5EPCh. 15 - What is the geometrical arrangement for the atoms...Ch. 15 - Prob. 15.7EPCh. 15 - Prob. 15.8EPCh. 15 - Prob. 15.9EPCh. 15 - Prob. 15.10EPCh. 15 - Prob. 15.11EPCh. 15 - Classify each of the following structures as an...Ch. 15 - Prob. 15.13EPCh. 15 - Prob. 15.14EPCh. 15 - Prob. 15.15EPCh. 15 - Prob. 15.16EPCh. 15 - Prob. 15.17EPCh. 15 - Prob. 15.18EPCh. 15 - Prob. 15.19EPCh. 15 - Prob. 15.20EPCh. 15 - Prob. 15.21EPCh. 15 - Prob. 15.22EPCh. 15 - Prob. 15.23EPCh. 15 - Prob. 15.24EPCh. 15 - Prob. 15.25EPCh. 15 - Prob. 15.26EPCh. 15 - Prob. 15.27EPCh. 15 - Prob. 15.28EPCh. 15 - Prob. 15.29EPCh. 15 - Prob. 15.30EPCh. 15 - Prob. 15.31EPCh. 15 - Prob. 15.32EPCh. 15 - Prob. 15.33EPCh. 15 - Prob. 15.34EPCh. 15 - Prob. 15.35EPCh. 15 - Prob. 15.36EPCh. 15 - Prob. 15.37EPCh. 15 - Prob. 15.38EPCh. 15 - Prob. 15.39EPCh. 15 - Prob. 15.40EPCh. 15 - Draw a structural formula for each of the...Ch. 15 - Prob. 15.42EPCh. 15 - Prob. 15.43EPCh. 15 - Prob. 15.44EPCh. 15 - Prob. 15.45EPCh. 15 - Prob. 15.46EPCh. 15 - Prob. 15.47EPCh. 15 - Prob. 15.48EPCh. 15 - Prob. 15.49EPCh. 15 - Prob. 15.50EPCh. 15 - Prob. 15.51EPCh. 15 - Prob. 15.52EPCh. 15 - Prob. 15.53EPCh. 15 - Prob. 15.54EPCh. 15 - Prob. 15.55EPCh. 15 - Prob. 15.56EPCh. 15 - Prob. 15.57EPCh. 15 - Prob. 15.58EPCh. 15 - Prob. 15.59EPCh. 15 - Prob. 15.60EPCh. 15 - Prob. 15.61EPCh. 15 - Prob. 15.62EPCh. 15 - Prob. 15.63EPCh. 15 - Prob. 15.64EPCh. 15 - Prob. 15.65EPCh. 15 - Prob. 15.66EPCh. 15 - Prob. 15.67EPCh. 15 - Which member in each of the following pairs of...Ch. 15 - Prob. 15.69EPCh. 15 - Prob. 15.70EPCh. 15 - Prob. 15.71EPCh. 15 - Prob. 15.72EPCh. 15 - Prob. 15.73EPCh. 15 - Prob. 15.74EPCh. 15 - Prob. 15.75EPCh. 15 - Prob. 15.76EPCh. 15 - Prob. 15.77EPCh. 15 - Prob. 15.78EPCh. 15 - Prob. 15.79EPCh. 15 - What is the chemical formula of the inorganic...Ch. 15 - Prob. 15.81EPCh. 15 - Which of the following compounds would react with...Ch. 15 - Prob. 15.83EPCh. 15 - Prob. 15.84EPCh. 15 - Which of the three compounds pentanal,...Ch. 15 - Prob. 15.86EPCh. 15 - Prob. 15.87EPCh. 15 - Prob. 15.88EPCh. 15 - Prob. 15.89EPCh. 15 - Prob. 15.90EPCh. 15 - Prob. 15.91EPCh. 15 - Prob. 15.92EPCh. 15 - Which carbon atom is the hemiacetal carbon atom in...Ch. 15 - Prob. 15.94EPCh. 15 - Prob. 15.95EPCh. 15 - Prob. 15.96EPCh. 15 - Prob. 15.97EPCh. 15 - Prob. 15.98EPCh. 15 - Prob. 15.99EPCh. 15 - Prob. 15.100EPCh. 15 - Prob. 15.101EPCh. 15 - Prob. 15.102EPCh. 15 - Prob. 15.103EPCh. 15 - Prob. 15.104EPCh. 15 - Prob. 15.105EPCh. 15 - Prob. 15.106EPCh. 15 - Prob. 15.107EPCh. 15 - Prob. 15.108EPCh. 15 - Prob. 15.109EPCh. 15 - Prob. 15.110EPCh. 15 - Prob. 15.111EPCh. 15 - Prob. 15.112EPCh. 15 - Prob. 15.113EPCh. 15 - Prob. 15.114EPCh. 15 - Prob. 15.115EPCh. 15 - Prob. 15.116EPCh. 15 - Prob. 15.117EPCh. 15 - Prob. 15.118EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forward
- Draw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forward
- An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forward
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning