
Concept explainers
What is the chemical formula of the inorganic product formed, if any, in each of the reactions in Problem 15-78?
- a. Propanal in the Tollens test
- b. 3-Pentanone in the Tollens test
- c. Methylpropanal in the Benedict’s test
- d. Propanone in the Benedict’s test
(a)
Interpretation:
The inorganic product formed when propanal undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
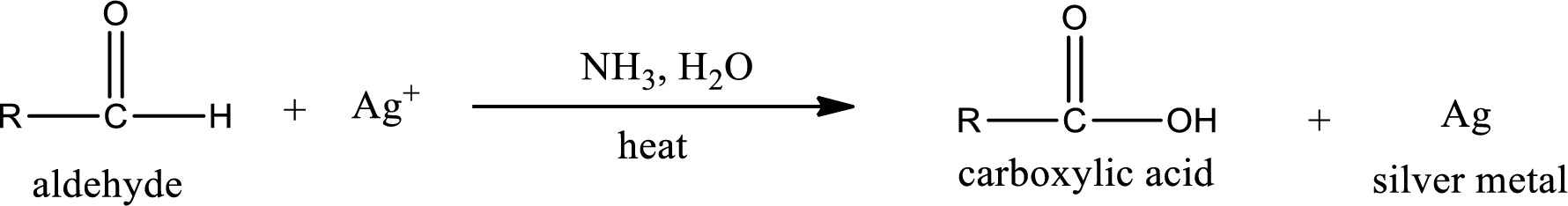
Ketone does not undergo Tollen’s test to deposit silver metal.
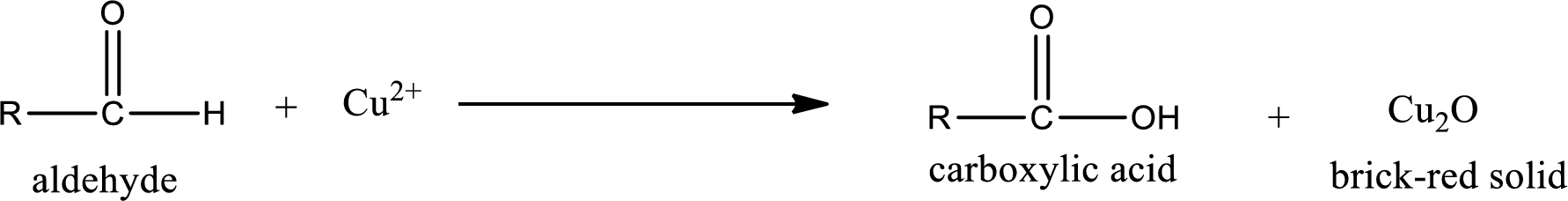
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is silver metal.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
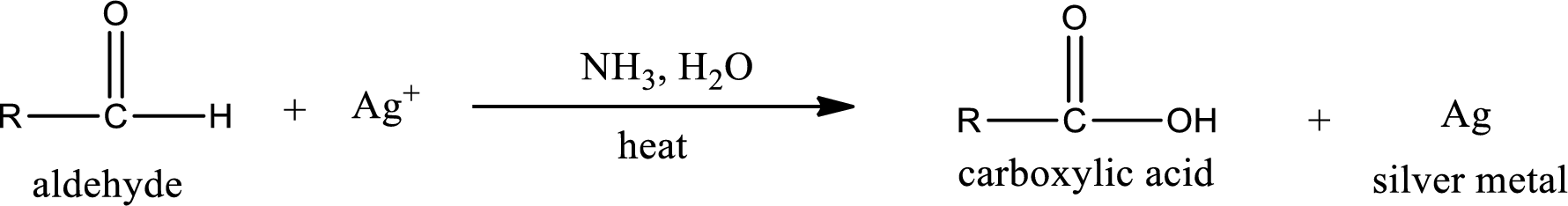
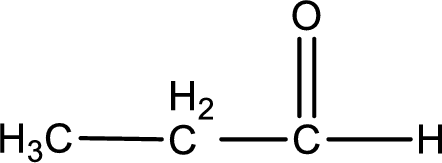
Given aldehyde is propanal and the structure can be given as shown below,
This on reaction with Tollen’s reagent gives carboxylic acid and silver metal as the product. The structure of the inorganic product formed and the complete reaction can be given as shown below,
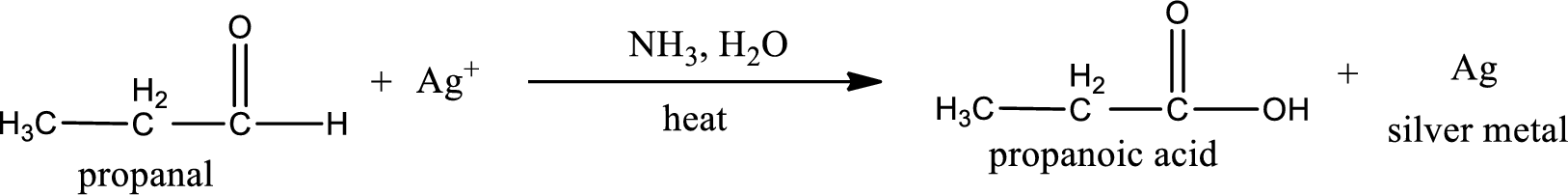
Silver metal is formed as the inorganic product when propanal undergoes Tollen’s test.
The inorganic product formed is given.
(b)
Interpretation:
The inorganic product formed when 3-pentanone undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
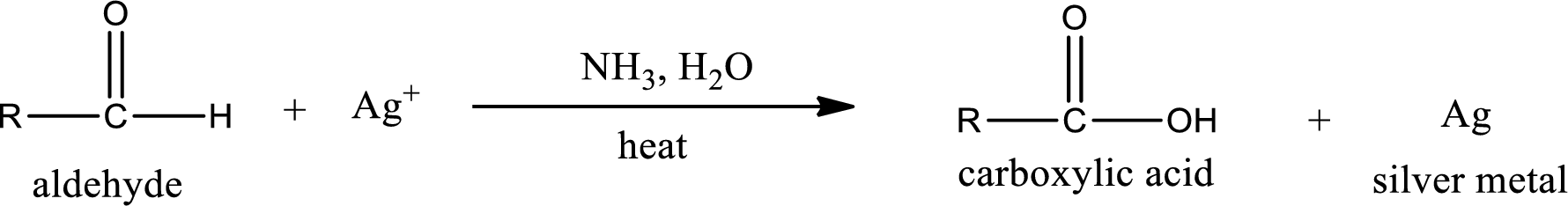
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is obtained as 3-pentanone does not undergo Tollen’s test.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
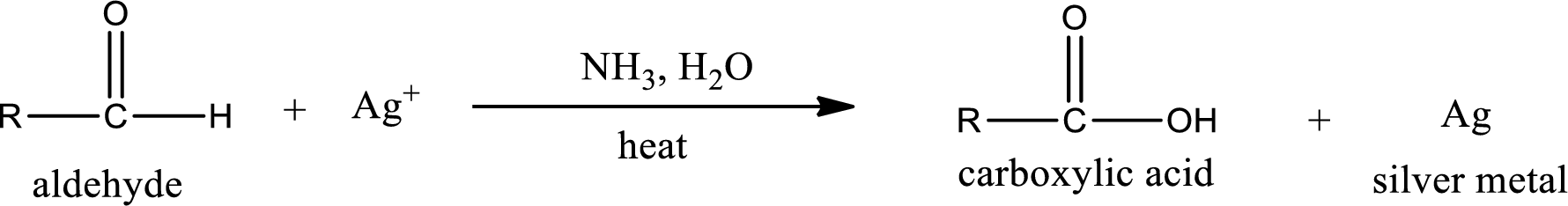
Given compound is a ketone that is 3-pentanone and the structure can be given as shown below,
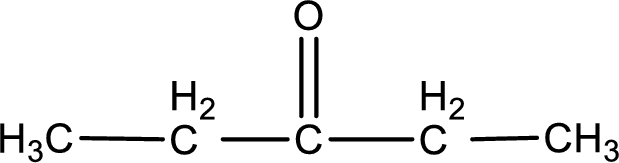
This on reaction with Tollen’s reagent does not give oxidized product. Therefore, no reaction takes place when 3-pentanone reacts with Tollen’s reagent.
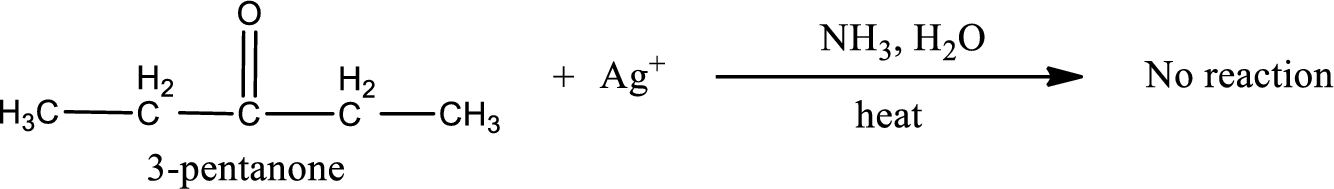
No inorganic product is formed when 3-pentanone undergoes Tollen’s test.
No reaction takes place when 3-pentanone undergoes Tollen’s test.
(c)
Interpretation:
The inorganic product formed when methylpropanal undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
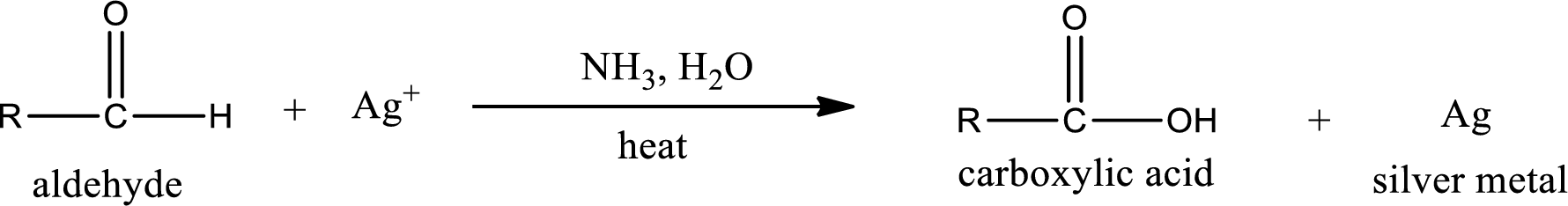
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
The inorganic product formed is
Explanation of Solution
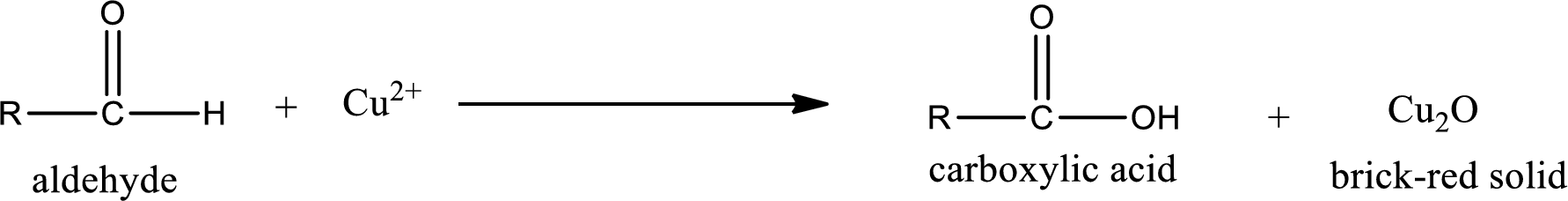
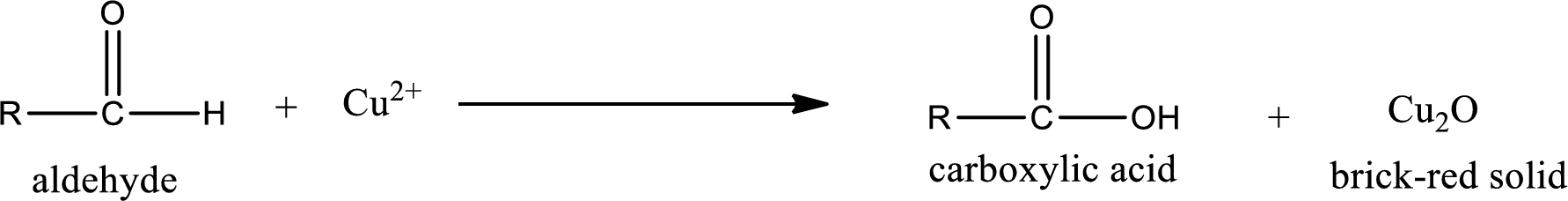
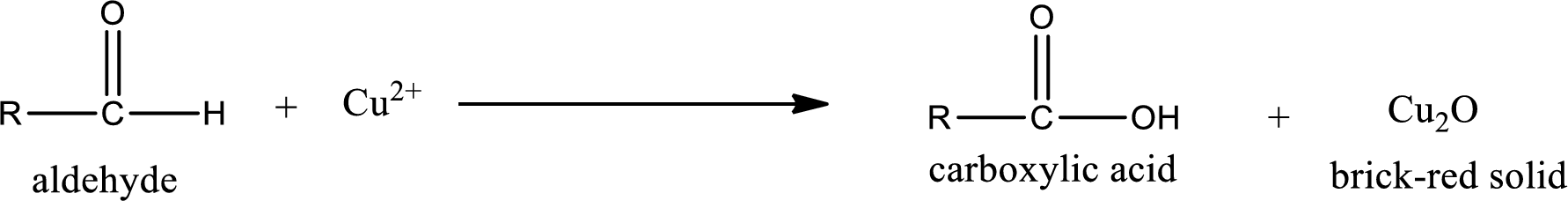
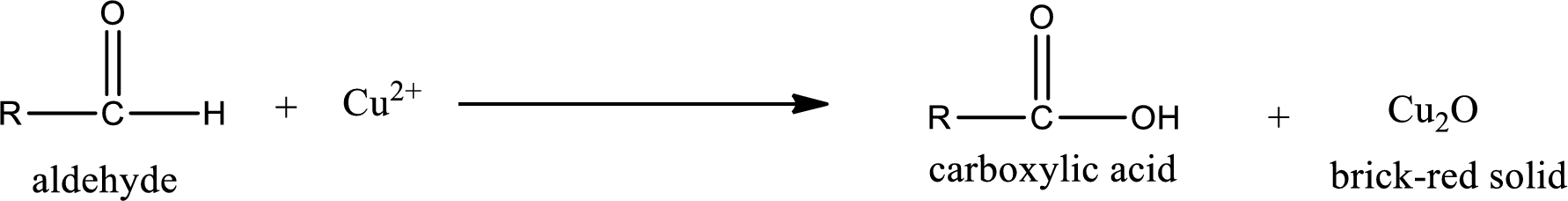
Aldehydes undergo Benedicts’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
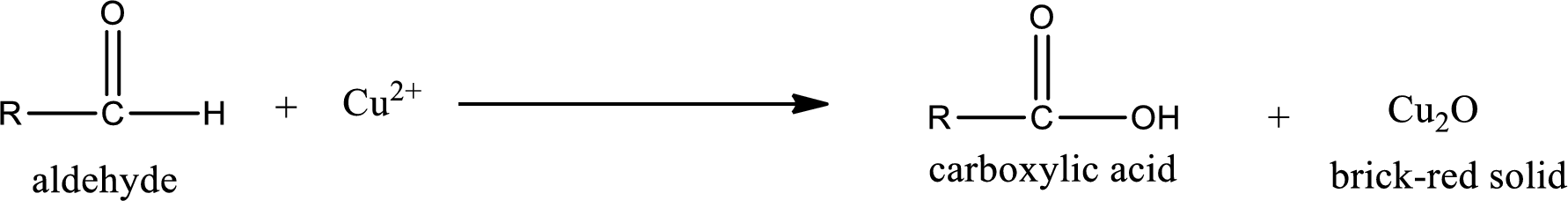
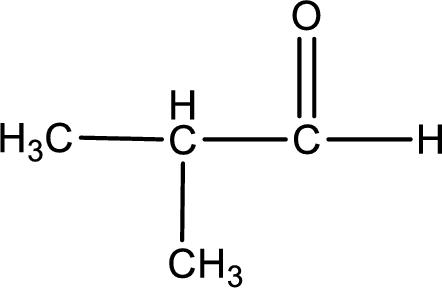
Given aldehyde is methylpropanal and the structure can be given as shown below,
This on reaction with Tollen’s reagent gives carboxylic acid and Copper(I) oxide as the product. The inorganic product formed and the complete reaction can be given as shown below,
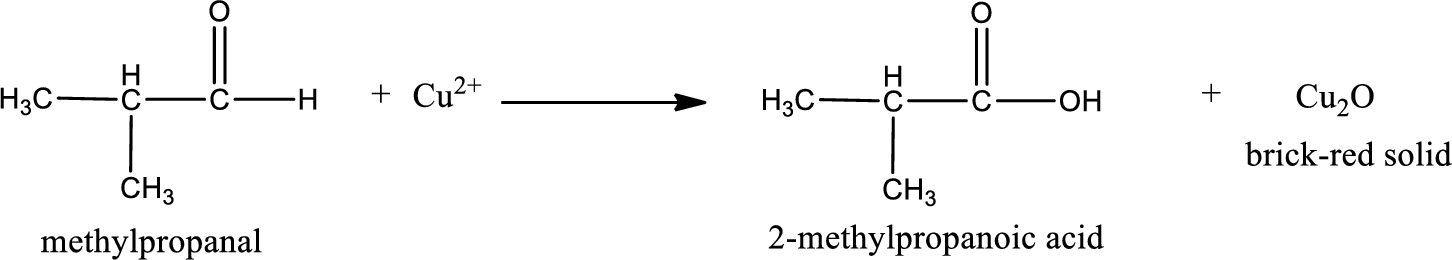
The inorganic product formed when methylpropanal undergoes Benedict’s test is given.
(d)
Interpretation:
The inorganic product formed when propanone undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
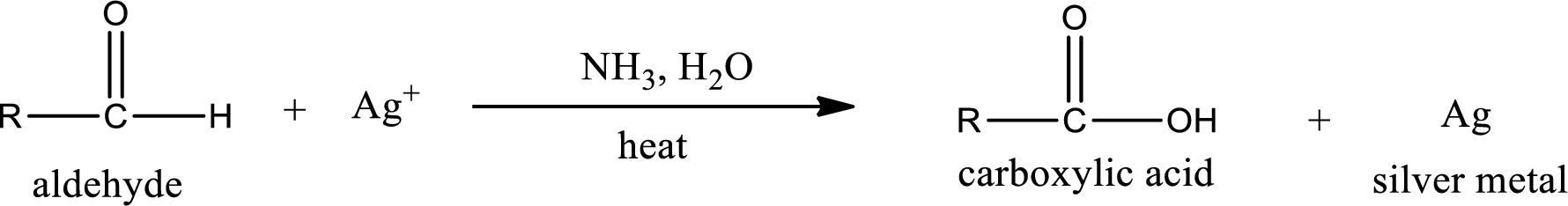
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
Answer to Problem 15.80EP
No inorganic product is formed when propanone undergoes Benedict’s test.
Explanation of Solution
Aldehydes undergo Benedict’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
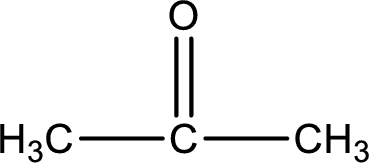
Given compound is a ketone. The name of ketone is propanone and the structure can be given as shown below,
This on reaction with Benedict’s reagent does not give oxidized product. Therefore, no reaction takes place when propanone undergoes Benedict’s test.
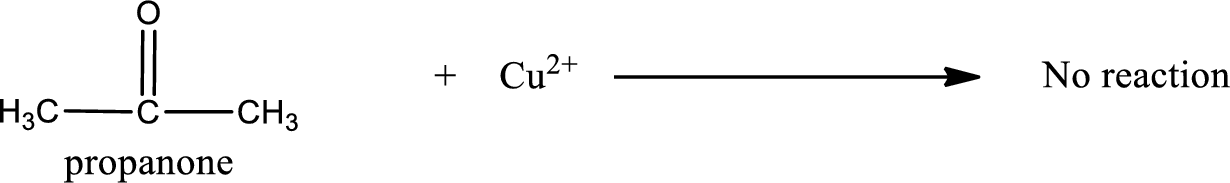
No inorganic product is formed when propanone undergo Benedict’s test.
No reaction takes place when propanone undergoes Benedict’s test.
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Use the literature Ka value of the acetic acid, and the data below to answer these questions. Note: You will not use the experimental titration graphs to answer the questions that follow. Group #1: Buffer pH = 4.35 Group #2: Buffer pH = 4.70 Group #3: Buffer pH = 5.00 Group #4: Buffer pH = 5.30 Use the Henderson-Hasselbalch equation, the buffer pH provided and the literature pKa value of acetic acid to perform the following: a) calculate the ratios of [acetate]/[acetic acid] for each of the 4 groups buffer solutions above. b) using the calculated ratios, which group solution will provide the best optimal buffer (Hint: what [acetate]/[acetic acid] ratio value is expected for an optimal buffer?) c) explain your choicearrow_forwardHow would you prepare 1 liter of a 50 mM Phosphate buffer at pH 7.5 beginning with K3PO4 and 1 M HCl or 1 M NaOH? Please help and show calculations. Thank youarrow_forwardDraw the four most importantcontributing structures of the cation intermediate thatforms in the electrophilic chlorination of phenol,(C6H5OH) to form p-chlorophenol. Put a circle aroundthe best one. Can you please each step and also how you would approach a similar problem. Thank you!arrow_forward
- A 100mM lactic acid/lactate buffer was found to have a lactate to lactic acid ratio of 2 and a pH of 4.2. What is the pKa of lactic acid? Can you please help show the calculations?arrow_forwardUsing line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?arrow_forwardUsing dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!arrow_forward
- 5) There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the hybridization scheme for the compound. (8) 10,11 7) 1.2.3 H 4 | 14 8) COC 12 13 H 16 15 H7 9) - 5.6 C 8 H 10) H 1). 2) 3)_ 11) 12) 13) 4)_ 14) 5) 15) 16) 6)arrow_forwardThe sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forwardConsider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





