
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.36CP
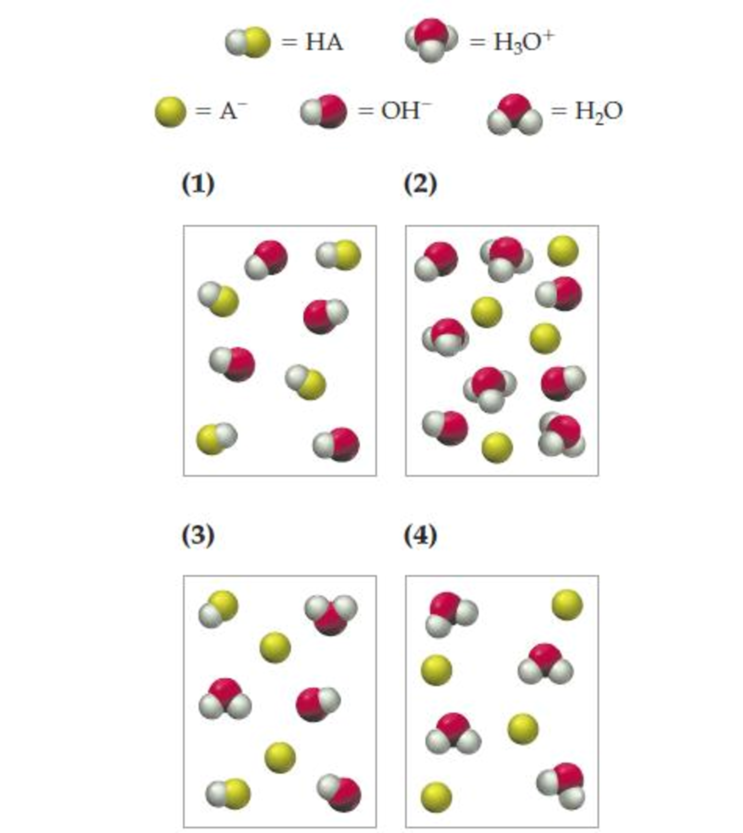
The strong acid HA is mixed with an equal molar amount of aqueous NaOH. Which of the following pictures represents the equilibrium state of the solution? (Na+ ions and solvent water molecules have been omitted for clarity.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
utron
eutro
cle
TH
tro
(Na
(b) Atoms are said to be electrically neutral. Explain.
(c)
Distinguish
between the following:
(i) Atomic number and mass number.
(ii) Mass number and relative atomic mass.
2. An isotope Q, has 18 neutrons a mass number of 34.
(a) (i) Draw the atomic structure of Q.
(ii) Write its electron arrangement
(b) To which period and group does Q belong? Explain your answer.
(c) How does Q form its ion? Explain.
3. (a) Determine the relative atomic mass of the following elements =
compositions occur in the proportions given.
(i) Neon
20
21
22.
Ne (90.92%), 10Ne (0.26%), and 10Ne (8.82%)
(ii) Argon
36
38
40
18 Ar (0.34%), 18 Ar (0.06%) and 18 Ar (99.6%)
In the normal hydrogen electrode, the balance potential difference in the interface is this, the maximum potential is 5 mV. Explain briefly.
The electrode balance potential is -0.118 V and the interface potential difference
is +5 mV. The overvoltage n will be 0.005 - (-0.118) = 0.123 V. Is it correct?
Chapter 15 Solutions
General Chemistry: Atoms First
Ch. 15.1 - Write balanced net ionic equations for the...Ch. 15.1 - Write balanced net ionic equations for the...Ch. 15.2 - Calculate the concentrations of all species...Ch. 15.2 - Calculate the pH in a solution prepared by...Ch. 15.2 - Prob. 15.5CPCh. 15.3 - The following pictures represent solutions that...Ch. 15.3 - Calculate the pH of 0.100 L of a buffer solution...Ch. 15.3 - Calculate the change in pH when 0.002 mol of HNO3...Ch. 15.4 - Use the HendersonHasselbalch equation to calculate...Ch. 15.4 - Prob. 15.10P
Ch. 15.4 - Suppose you are performing an experiment that...Ch. 15.4 - Prob. 15.12PCh. 15.6 - A 40.0 mL volume of 0.100 M HCl is titrated with...Ch. 15.6 - A 40.0 mL volume of 0.100 M NaOH is titrated with...Ch. 15.7 - The following pictures represent solutions at...Ch. 15.7 - Consider the titration of 100.0 mL of 0.016 M HOCl...Ch. 15.7 - The following acid-base indicators change color in...Ch. 15.9 - Assume that 40.0 mL of 0.0800 M H2SO3 (Ka1 = 1.5 ...Ch. 15.9 - Assume that 40.0 mL of a 0.0250 M solution of the...Ch. 15.10 - Write the equilibrium-constant expression for Ksp...Ch. 15.11 - A saturated solution of Ca3(PO4)2 has [Ca2+] =...Ch. 15.11 - Prob. 15.22PCh. 15.11 - Which has the greater molar solubility: AgCl with...Ch. 15.11 - Prob. 15.24CPCh. 15.12 - Calculate the molar solubility of MgF2 in 0.10 M...Ch. 15.12 - Which of the following compounds are more soluble...Ch. 15.12 - In an excess of NH3(aq), Cu2+ ion forms a deep...Ch. 15.12 - Silver bromide dissolves in aqueous sodium...Ch. 15.13 - Prob. 15.29PCh. 15.13 - Will a precipitate form on mixing 25 mL of 1.0 ...Ch. 15.14 - Prob. 15.31PCh. 15.15 - Prob. 15.32PCh. 15 - The following pictures represent solutions that...Ch. 15 - The following pictures represent solutions that...Ch. 15 - The strong acid HA is mixed with an equal molar...Ch. 15 - The following pictures represent solutions at...Ch. 15 - The following pictures represent solutions at...Ch. 15 - The following pictures represent solutions at...Ch. 15 - Prob. 15.40CPCh. 15 - Prob. 15.41CPCh. 15 - Prob. 15.42CPCh. 15 - Prob. 15.43CPCh. 15 - Is the pH greater than, equal to, or less than 7...Ch. 15 - Prob. 15.45SPCh. 15 - Which of the following mixtures has the higher pH?...Ch. 15 - Which of the following mixtures has the lower pH?...Ch. 15 - Phenol (C6H5OH, Ka = 1.3 1010) is a weak acid...Ch. 15 - Aniline (C6H5NH2, Kb = 4.3 1010) is a weak base...Ch. 15 - The equilibrium constant Kn for the neutralization...Ch. 15 - The equilibrium constant Kn for the neutralization...Ch. 15 - Prob. 15.52SPCh. 15 - Does the pH increase, decrease, or remain the same...Ch. 15 - Prob. 15.54SPCh. 15 - Calculate the pH of a solution prepared by mixing...Ch. 15 - Prob. 15.56SPCh. 15 - The pH of a solution of NH3 and NH4Br is 8.90....Ch. 15 - Prob. 15.58SPCh. 15 - Prob. 15.59SPCh. 15 - Prob. 15.60SPCh. 15 - Which of the following gives a buffer solution...Ch. 15 - Prob. 15.62SPCh. 15 - Prob. 15.63SPCh. 15 - Calculate the pH of a buffer solution that is 0.20...Ch. 15 - Prob. 15.65SPCh. 15 - Calculate the pH of 0.250 L of a 0.36 M formic...Ch. 15 - Calculate the pH of0.375 L of a 0.18 M acetic...Ch. 15 - Prob. 15.68SPCh. 15 - Use the HendersonHasselbalch equation to calculate...Ch. 15 - Prob. 15.70SPCh. 15 - Give a recipe for preparing a CH3CO2HCH3CO2Na...Ch. 15 - Prob. 15.72SPCh. 15 - Prob. 15.73SPCh. 15 - What is the Ka of the amino acid leucine if it is...Ch. 15 - Prob. 15.75SPCh. 15 - Prob. 15.76SPCh. 15 - Make a rough plot of pH versus milliliters of acid...Ch. 15 - Prob. 15.78SPCh. 15 - Consider the titration of 50.0 mL of 0.116 M NaOH...Ch. 15 - Consider the titration of 40.0 mL of 0.250 M HF...Ch. 15 - A 100.0 mL sample of 0.100 M methylamine (CH3NH2,...Ch. 15 - Prob. 15.82SPCh. 15 - Consider the titration of 25.0 mL of 0.0200 M...Ch. 15 - Prob. 15.84SPCh. 15 - The equivalence point was reached in titrations of...Ch. 15 - Prob. 15.86SPCh. 15 - What is the pH at the equivalence point for the...Ch. 15 - Prob. 15.88SPCh. 15 - Prob. 15.89SPCh. 15 - Prob. 15.90SPCh. 15 - Prob. 15.91SPCh. 15 - Prob. 15.92SPCh. 15 - Prob. 15.93SPCh. 15 - Prob. 15.94SPCh. 15 - Prob. 15.95SPCh. 15 - Prob. 15.96SPCh. 15 - Prob. 15.97SPCh. 15 - Use Le Chteliers principle to explain the...Ch. 15 - Use Le Chteliers principle to predict whether the...Ch. 15 - Calculate the molar solubility of PbCrO4 in:...Ch. 15 - Calculate the molar solubility of SrF2 in:...Ch. 15 - Which of the following compounds are more soluble...Ch. 15 - Which of the following compounds are more soluble...Ch. 15 - Prob. 15.104SPCh. 15 - Is the solubility of Fe(OH)3 increased, decreased,...Ch. 15 - Prob. 15.106SPCh. 15 - Prob. 15.107SPCh. 15 - Prob. 15.108SPCh. 15 - Prob. 15.109SPCh. 15 - Calculate the molar solubility of AgI in: (a)Pure...Ch. 15 - Calculate the molar solubility of Cr(OH)3 in 0.50...Ch. 15 - What compound, if any, will precipitate when 80 mL...Ch. 15 - Prob. 15.113SPCh. 15 - Prob. 15.114SPCh. 15 - In qualitative analysis, Al3+ and Mg2+ are...Ch. 15 - Prob. 15.116SPCh. 15 - Can Co2+ be separated from Zn2+ by bubbling H2S...Ch. 15 - Prob. 15.118SPCh. 15 - Prob. 15.119SPCh. 15 - Prob. 15.120SPCh. 15 - Give a method for separating the following pairs...Ch. 15 - Assume that you have three white solids: NaCl,...Ch. 15 - On the same graph, sketch pH titration curves for...Ch. 15 - Prob. 15.124CHPCh. 15 - Prob. 15.125CHPCh. 15 - A saturated solution of Mg(OH)2 in water has pH =...Ch. 15 - Prob. 15.128CHPCh. 15 - In qualitative analysis, Ag+, Hg22+, and Pb2+ are...Ch. 15 - Calculate the molar solubility of MnS in a 0.30 M...Ch. 15 - Prob. 15.131CHPCh. 15 - Prob. 15.132CHPCh. 15 - Prob. 15.133CHPCh. 15 - Prob. 15.134CHPCh. 15 - Prob. 15.135CHPCh. 15 - A 100.0 mL sample of a solution that is 0.100 M in...Ch. 15 - A 0.0100 mol sample of solid Cd(OH)2 (Ksp = 5.3 ...Ch. 15 - Zinc hydroxide, Zn(OH)2 (Ksp = 4.1 1017), is...Ch. 15 - Prob. 15.139CHPCh. 15 - Prob. 15.140MPCh. 15 - Ethylenediamine (NH2CH2CH2NH2, abbreviated en) is...Ch. 15 - A 40.0 mL sample of a mixture of HCl and H3PO4 was...Ch. 15 - A 1.000 L sample of HCl gas at 25 C and 732.0 mm...Ch. 15 - Prob. 15.144MPCh. 15 - Consider the reaction that occurs on mixing 50.0...Ch. 15 - In qualitative analysis, Ca2+ and Ba2+ are...Ch. 15 - A railroad tank car derails and spills 36 tons of...Ch. 15 - Prob. 15.148MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the electrode Pt, H2(1 atm) | H+(a=1), if the electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The current voltage will be 0.005 - (-0.118) = 0.123 V ¿Correcto?arrow_forwardIn the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.arrow_forwardIn one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.arrow_forward
- In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forward
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY